Int. J. Dev. Biol. 67: 79 - 90 (2023)
Polarized contact behavior in directionally migrating Xenopus gastrula mesendoderm
Open Access | Original Article | Published: 18 September 2023
Abstract
The control of cell-cell adhesion and detachment is essential for collective migration and cell rearrangement. Here, we have used the contact behavior of Xenopus gastrula mesoderm explants migrating directionally on ectoderm conditioned substratum to study the regulation of active cell-cell detachment. When colliding laterally, explants repelled each other, whereas they fused front-to-back when aligned in the direction of migration. For this mesoderm polarization by the substratum, we identified three control modules. First, PDGF-A signaling normally suppresses contact-induced collapse of lamellipodia in a polarized manner. Disruption of PDGF-A function, or of Xwnt6, decreased the polarization of explant contact behavior. Second, the Wnt receptor Xfz7 acted upstream of the kinase Pak1 to control explant fusion independently of PDGF-A-promoted lamellipodia stability. Third, ephrinB1 acted with Dishevelled (Dvl) in front-to-back explant fusion. The second and third modules have been identified previously as regulators of tissue separation at the ectoderm-mesoderm boundary. On non-polarizing, fibronectin-coated substratum, they controlled repulsion between explants in the same way as between tissues during boundary formation. However, explant repulsion/fusion responses were reversed on conditioned substratum by the endogenous guidance cues that also control oriented contact inhibition of lamellipodia. Together, control modules and substratum-bound guidance cues combine preferential front-back adhesion and diminished lateral adhesion of cells to promote collective directional mesoderm migration.
Keywords
gastrula, mesoderm, cell migration, tissue separation, cell repulsion
Introduction
Collective cell migration, as well as the rearrangement of cells by differential migration, are basic morphogenetic processes, and the control of cell contact dynamics is essential in respective tissue movements. While adhesion must be maintained overall to ensure tissue cohesion, cells must also detach and reattach in an orderly manner to exchange neighbors. Detachment can result from cells being gradually peeled off each other as they move apart, but it can also be an active, regulated process which, organized in space and time, generates specific intercalation and migration patterns.
In the Xenopus gastrula, prechordal mesoderm cells are gradually separated by peeling during Prickle-1-dependent radial intercalation in the prechordal mesoderm (Huang and Winklbauer, 2022). When the process is studied in vitro, most cadherins are removed from a contact area by disrupting their trans bonds and the molecules diffusing onto the adjacent free cell surface. Remaining cadherins are concentrated at the shrinking contacts until eventually their link with the actin cytoskeleton breaks and the contact is resolved (Rozema et al., 2023). A gradual peeling apart of adjacent endoderm cells also accompanies the cell rearrangements of vegetal rotation. It requires ephrinB1-dependent resorption of cell-cell contacts by trogocytosis and of free surface by macropinocytosis (Wen and Winklbauer, 2017; Gong et al., 2019).
Examples of active detachment processes have also been studied in the Xenopus gastrula. In leading edge mesendoderm (LEM), lamellipodia that attempt to move across the surfaces of adjacent cells are generally induced to detach and collapse, and this contact inhibition of lamellipodia (CILa) is mediated, among other factors like C-cadherin or integrinβ1, by the serine/threonine kinase Pak1 and the Eph receptor ligand ephrinB1 (Nagel and Winklbauer, 2018). However, as the LEM cells move collectively towards the animal pole of the embryo, they extend lamellipodia in the direction of overall movement and underlap each other to form a “shingle arrangement” (Winklbauer and Nagel, 1991; Nagel et al., 2004, 2009, 2021). This orientation depends on the spatial regulation of lamellipodia collapse by ectodermal PDGF-A (Ataliotis et al., 1995) that is secreted into the extracellular matrix of the substratum. In LEM cells, the receptor PDGFRα is enriched at the animally pointing side, irrespective of lamellipodia orientation. Only when a random protrusion extends animally is it aligned with the receptor gradient of the cell, receives it a strong PDGF-A signal, and is it protected from CILa. When protrusions extend laterally or vegetally by chance they detach and retract rapidly (Nagel and Winklbauer, 2018).
Another well-studied example of controlled cell detachment is the formation of the ectoderm-mesoderm tissue boundary. In this process, asymmetric ephrin/Eph receptor signaling generates high actomyosin cortical tension on the ectodermal side of the tissue interface, which straightens the boundary and prevents cell mixing (Canty et al., 2017; Barua and Winklbauer, 2022). In addition to this, Eph/ephrin signaling promotes the repulsion of mesoderm cells by the ectoderm. Repulsion is triggered by heterotypic contact between mesoderm and ectoderm cell bodies. This allows the membrane-bound ephrin ligands to interact with the Eph transmembrane receptors and leads to the rapid retraction of the mesoderm cell surface. Contact is re-established after a few minutes, followed by another bout of repulsion, thus generating repeating cycles of adhesion and detachment (Rohani et al., 2011). Lamellipodia are not affected by this cell body-cell body interaction and the shingle arrangement is maintained in the LEM during detachment (Nagel et al., 2021), implying independent control mechanisms for cell body repulsion and CILa.
Directional migration of LEM explants can be studied in vitro on conditioned substratum (CS), a surface coated with the matrix and guidance cues of the ectodermal BCR (Nakatsuji and Johnson, 1983; Winklbauer and Nagel, 1991; Nagel and Winklbauer, 1999). When several LEM explants were placed on a single CS (see Fig. 1A), we noted that explants became polarized with respect to their contact behavior upon collision, such that they fused in one direction and were repelled in the other. Explant collision on CS provides a simple assay to study polarized LEM contact behavior during directional migration. We found that this behavior is intricately related to both the contact inhibition of lamellipodia and the repulsion of cell bodies.
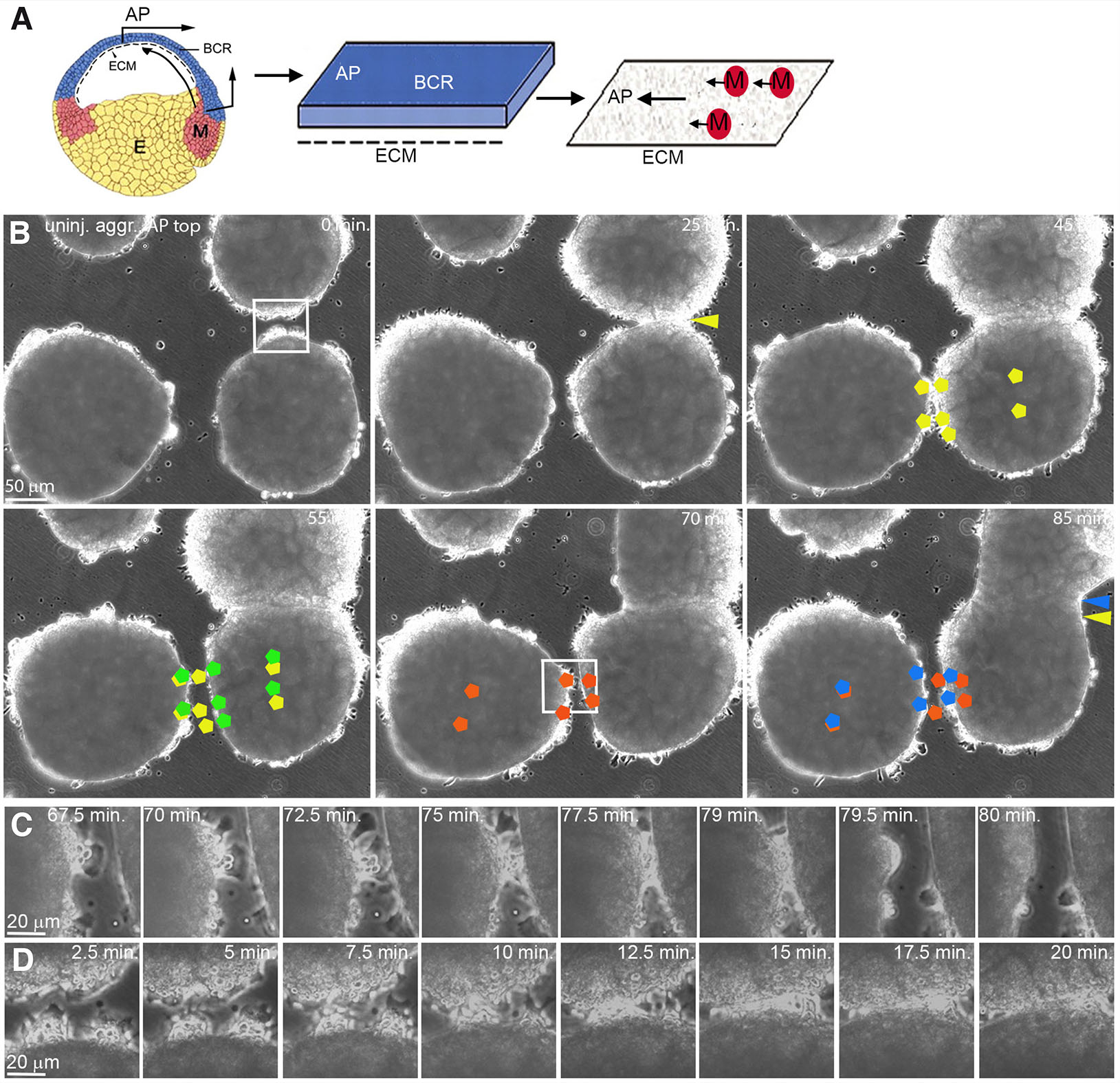
Fig. 1. Polarized, contact-induced margin retraction of LEM explants on CS.
(A) CS preparation. A blastocoel roof (BCR) explant extending to the animal pole (AP) is cultured with its inner side down. After removal, LEM explants move preferentially to the AP (black arrows) on the CS. (B) Frames from time-lapse recordings showing polarized retraction of LEM explant margins, AP to the top. Yellow and green pentagons indicate initial and post-retraction positions, respectively, of cells during a lateral explant encounter. Margin of right explant retracts; its center shifts animally. Red and blue pentagons, initial and post-retraction positions of cells during another lateral encounter. Left explant margin retracts, explant center remains in place. Yellow and blue arrow heads, initial and final positions of fusion sutures. (C,D) Higher magnification, lateral repulsion (C) and front-back fusion (D). Abbreviations: CS, conditioned substratum; E, endoderm; ECM, extracellular matrix; M, LEM; LEM, leading edge mesendoderm.
Results
Contact-induced margin retraction of directionally migrating LEM explants is polarized
To examine the contact behavior of directionally migrating LEM explants, several explants were placed on the same CS (Fig. 1A). Lamellipodia in the interior of explants become oriented in the direction of translocation (Winklbauer et al., 1992; Nagel and Winklbauer, 2018). Strikingly, contact behavior of whole explants is also polarized (Fig. 1B; movie 1). Lateral margins of explants that move parallel to each other to the animal pole repel each other and retract upon contact. Retraction is local, and explants do not migrate apart as a whole. In addition, margins do not retract immediately but only when several cells have been in contact for several minutes (Fig. 1C). By contrast, front-back encounters of LEM explants lead to explant fusion where margins adhere to each other until the suture between former explants eventually disappears (Fig. 1 B,D).
To relate explant repulsion/fusion to lamellipodia behavior, we followed labelled cells in time-lapse recordings. Although cells in the interior of directionally migrating LEM explants are oriented with their lamellipodia towards the animal pole, marginal cells extend protrusions away from the explant onto the free substratum surface all around. At the front, marginal but also interior cells point animally as expected (Fig. 2A). However, at the back side of explants, cells at the free margin and in an adjacent row of interior cells point vegetally, against the direction of overall movement (Fig. 2B), and lateral cells similarly extend lamellipodia laterally (Fig. 2C). Apparently, the margin exerts an effect that is dominant over the orienting cues. In all cases, individual cells remain unipolar.
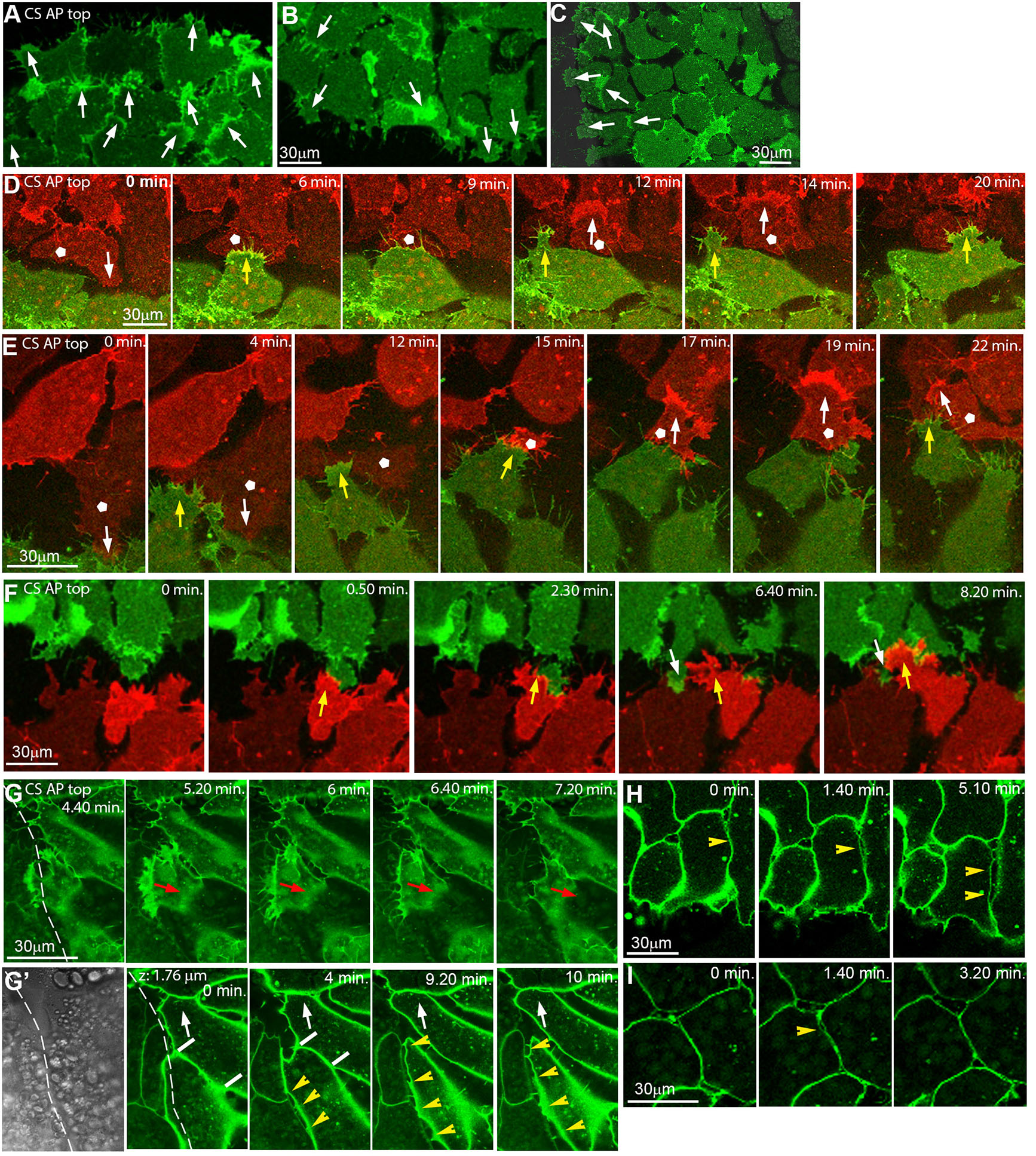
Fig. 2. Inverted microscope images showing polarized repulsion/fusion on CS at cell level.
(A-C) LEM explant margins, cells labelled with membrane-GFP, AP is to the top. At the front (A), lamellipodia of marginal and submarginal cells point animally; at the back (B) and laterally (C), marginal and some submarginal cells in row behind extend outward onto free substratum. Arrows, lamellipodia orientation. (D-F) Front-back interaction on CS of explants differently labelled with m-RFP (red) and m-GFP (green), from time-lapse recordings. Yellow arrows, front cells underlapping back cells. White pentagons, back cells that change lamellipodia orientation after contact (white arrows in D,E). Occasionally a back cell transiently underlaps front cells (white arrows in F). (G,G’) Time-lapse recording of explant lateral contact (dashed line) at substratum level (G) and 1.8 μm above (G’; first frame, transmitted light). Red arrows, lamellipodium retracting from boundary. White arrows, animally moving cell gradually shortens contact with other cell (white bars) to peel off. Yellow arrowheads, cell detachment by repulsion at lateral boundary. (H,I) Lateral, localized, transient cell-cell repulsion (yellow arrowheads) above substratum level. Abbreviations: CS, conditioned substratum; AP, animal pole; LEM, leading edge mesendoderm.
Fusion involves the underlapping of the rear cells of a leading explant by the front cells of the following explant, without induced retraction of lamellipodia (Fig. 2 D-F). In rare cases of back-to-front underlapping, lamellipodia are unstable and short-lived (Fig. 2F). Intriguingly, rear cells with protrusions pointing opposite the overall direction of movement become reoriented within minutes of contact, to align with explant movement (Fig. 2 D,E; movie2), completing fusion of the explants. Lamellipodia that point laterally retract upon contact to an adjacent explant, which prevents underlapping (Fig. 2G). Overall, the lamellipodia repulsion/stabilization pattern is reminiscent of the oriented contact inhibition of lamellipodia in the interior of directionally migrating explants, where lateral but not anterior protrusions are prone to collapse (Nagel and Winklbauer, 2018).
Above the substratum, rapid detachment of cell bodies that come into contact is observed, maintaining a cleft-like lateral boundary (Fig. 2G’, movie3). This is different from a slow, gradual peeling apart of cells further away from the boundary (Fig. 2G’), but there, abrupt lateral detachment occurs also occasionally. It is characterized by a rapid separation of cells over a large part of their contact, with thin filiform bridges remaining until the cells reattach again (Fig. 2 H,I). Thus, detachment by apparent repulsion is not limited to lamellipodia interactions but occurs also between cell bodies. Moreover, the polarization of whole-explant repulsion is independent of lamellipodia orientation in the respective marginal cells.
Polarized contact behavior of explants depends on PDGF-A/Xwnt6-mediated CS polarization
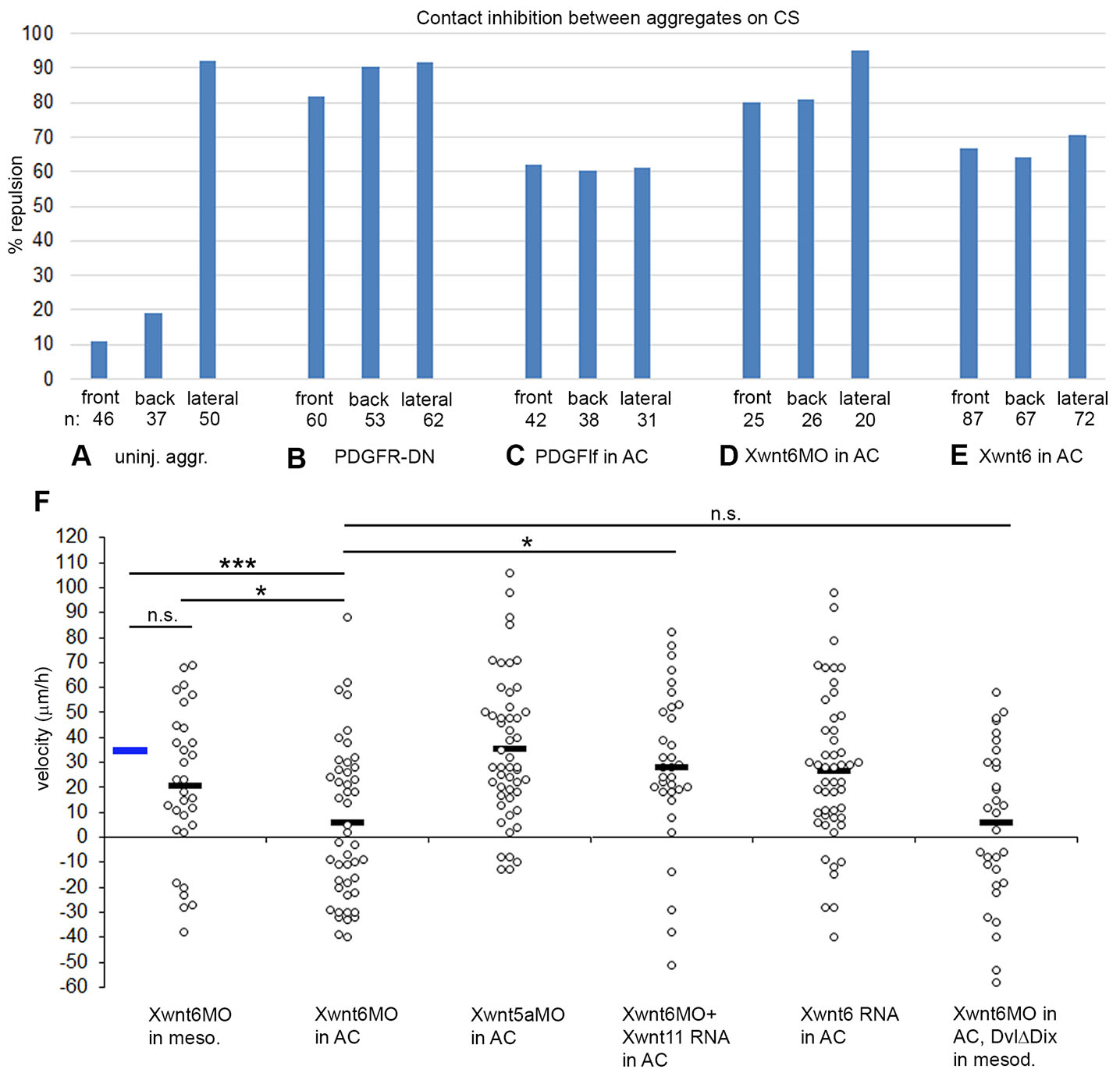
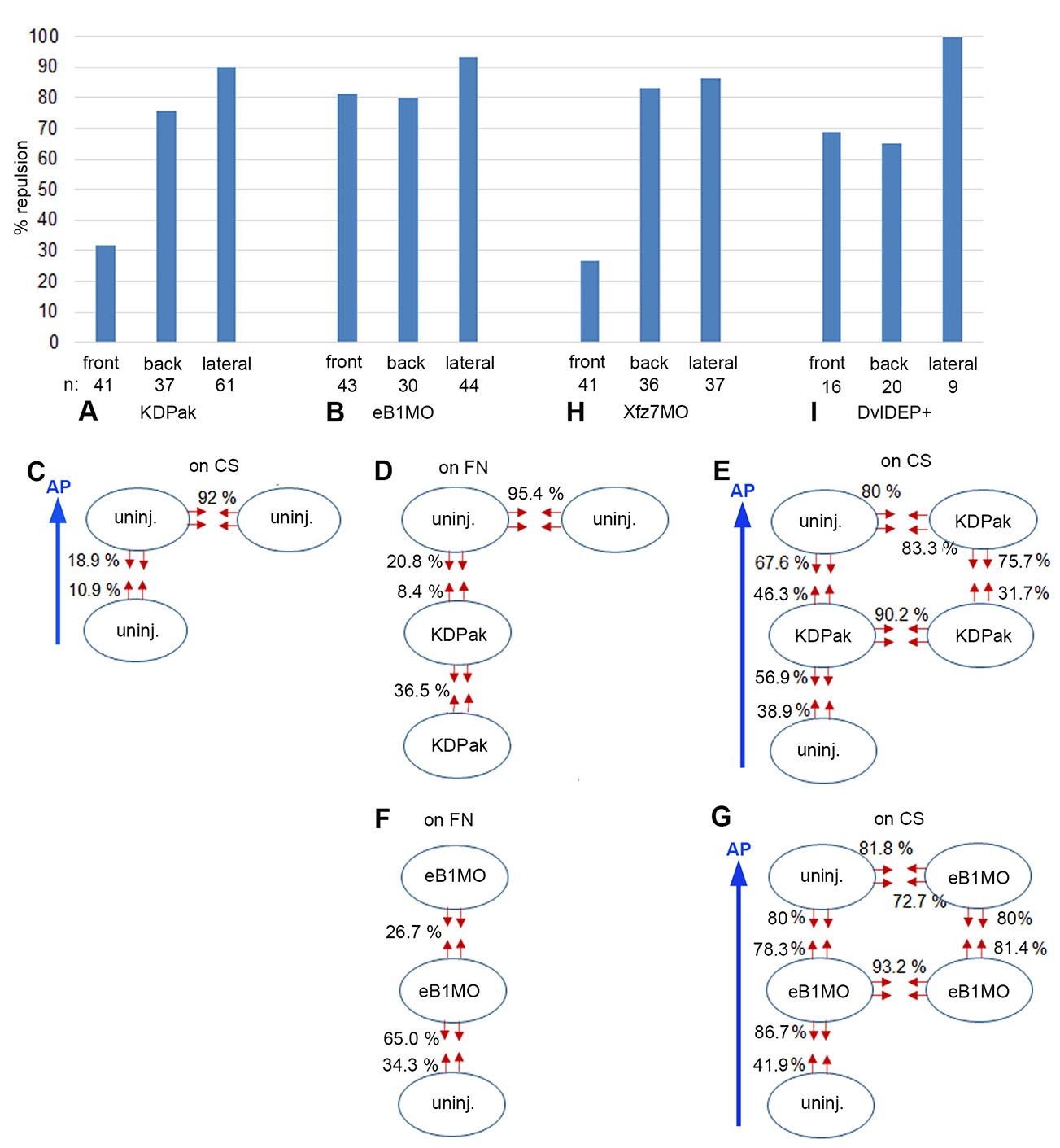
Between explants, almost all lateral contacts led to retraction while only 10% of front and 20% of back margins receded in front-to-back encounters (Fig. 3A). To see how contact behavior is regulated, we first examined PDGF-A, a factor involved in CILa polarization. We had shown that in explants expressing dominant-negative PDGFRα receptor, CILa is not suppressed directionally on CS as normally, and the few protrusions that attempt to form point in random directions (Nagel and Winklbauer, 2018). Under these conditions, lateral, front and back margins retract about as strongly as lateral contacts do in uninjected explants (Fig. 3B), consistent with lacking suppression of contact inhibition. When ectoderm that overexpresses matrix-binding lf-PDGF-A produces CS, CILa is strongly suppressed and lamellipodia are stabilized even when pointing in aberrant directions (Nagel and Winklbauer, 2018). Hence, explant margins should be less repellent on all sides. Repulsion is indeed evenly reduced compared to that which occurs in PDGF signaling-impaired explants, but this involves reduced lateral and increased front/back repulsion (Fig. 3C). Thus, explant margins respond qualitatively to PDGF-A signaling as expected from previously described contact inhibition of interior lamellipodia, but additional factors must modify their reaction.
Fig. 3. Margin repulsion/fusion and directional migration of LEM explants on conditioned substratum.
(A-E) Percentage of encounters resulting in repulsion at front, back and lateral sides of explants. (A) Uninjected explants; (B) LEM explants expressing dominant negative receptor, PDGFRα; (C) substratum conditioned with BCR overexpressing lf-PDGF-A, (D) with Xwnt6 depleted, and (E) with Xwnt6 overexpressing BCR. (F) Directional migration of LEM explants on conditioned substratum with treatments as indicated. Velocities of explants towards the animal pole (positive values) or away (negative values) for each explant (dot) is indicated, black bars indicate averages. Blue bar, average of uninjected control (from Fig. 5A). ns, not significant; *, p ≤ 0.05; ***, p ≤ 0.001.
The Wnt receptor Xfz7 is involved in repulsion behaviors (see below), and in search for putative ligands we tested likely candidates Xwnt5a, Xwnt6 and Xwnt11. Unexpectedly, we noted that contact behavior of LEM explants on Xwnt6-modulated CS closely resembled that caused by altered PDGF-A signaling (Fig. 3 D,E). Upon Xwnt6 knockdown, front and back repulsion is strongly increased, and repulsion is maintained laterally (Fig. 3D). Overexpression of Xwnt6 in the ectodermal BCR, like overexpression of PDGF-A, abolished polarity: repulsion was equal at front, back, and lateral margins (Fig. 3E). This argues for Xwnt6 and PDGF-A acting in the same pathway. Consistent with this, directionality of LEM migration was abolished when Xwnt6 was knocked down in ectoderm used for substratum conditioning, but not when depleted in LEM explants on normal CS (Fig. 3F). Knockdown of Xwnt5a in BCR did not diminish directionality, but Xwnt6 depletion was rescued by Xwnt11, indicating limited specificity of the Wnt ligand requirement. Overexpression of Xwnt6 in BCR affected repulsion (Fig. 3E) but unlike lf-PDGF-A overexpression, it did not abrogate directionality. Lastly, Xwnt6 depletion in BCR could not be rescued by overexpression of DvlΔDIX in migrating LEM explants. This is consistent with the Xwnt6 ligand not interacting with a mesodermal Wnt receptor but functioning within the ectoderm where it could be necessary for proper PDGF-A deployment. Its overexpression effects could be too mild to reproduce those of direct lf-PDGF-A overexpression in the migration assay.
Pak1 and ephrinB1 control contact polarity independently of their role in CILa
In the LEM, Pak1 regulates CILa via ephrinB1 (Nagel et al., 2009; Nagel and Winklbauer, 2018), and these factors also control overall LEM polarity (Fig. 4 A,B). On non-polarizing fibronectin substratum, expression of kinase-dead, dominant-negative Pak1 (KD-Pak1) allows to reproduce in LEM explants contact behavior normally seen on CS (Fig. 4 C,D). Untreated explants repel each other strongly on fibronectin, like lateral neighbors on CS. Repulsion strength between KD-Pak1 explants is intermediate, but in heterotypic combinations of normal and KD-Pak1 explants, repulsion is strongly reduced, in uninjected less than in injected explants, mimicking the front and back explant behaviors seen on CS, respectively. This implies that Pak1 activity should be low at the front and high at the back side for fusion to occur.
Fig. 4. Margin repulsion/fusion of LEM explants on conditioned substratum.
(A,B,H,I) Percentage of encounters resulting in repulsion at front, back and lateral sides of LEM explants on conditioned substratum (CS). (A) KD-Pak1 expressing, (B) ephrinB1-depleted (eB1MO), (H) Xfz7-depleted and (I) Dvl-DEP+ expressing LEM explants. (C-E) Uninjected and KD-Pak1 expressing LEM explants (ovals) on CS (C,E) or on fibronectin (FN) substratum (D). Percentage of contact-induced retractions (red arrows) is indicated. (F,G) EphrinB1 depleted LEM explants on fibronectin (FN) substratum (F) or on CS (G). Retractions indicated as in (C-E). At n = 41 and n = 46, respectively, the front retraction frequency in KD-Pak1/KD-Pak1 encounters (31.7%) (E) is significantly higher at α = 0.01 than the corresponding frequency in controls (10.9%) (C), indicating a supportive role of Pak1 in front-back fusion. Blue arrows, direction of animal pole (AP).
Although Pak1-dependent repulsion/fusion on fibronectin mimics LEM contact behaviors on CS, the pattern is profoundly changed on CS (Fig. 4 A,E). Laterally, full repulsion in KD-Pak1/KD-Pak1 homotypic but also in KD-Pak1/uninjected heterotypic contacts suggests regulation independent of Pak1. In front-back interactions, asymmetry is retained upon Pak1 inhibition, but repulsion is increased in all explant combinations (Fig. 4E). This reveals a supportive role of Pak1 in front-back explant fusion on CS, which is the opposite of the repelling function on fibronectin, and inconsistent with Pak1 effects being mediated through CILa.
EphrinB1 promotes CILa downstream of Pak1, and its knockdown increases protrusion survival in LEM explants (Nagel and Winklbauer, 2018). On fibronectin, repulsion between ephrinB1-depleted explants is much reduced compared to untreated explants (Fig. 4F), which would be consistent with diminished CILa. However, contacts between ephrinB1-depleted and non-injected explants show only weakly reduced mutual repulsion, in stark contrast to Pak1/KD-Pak1 heterotypic contacts. Moreover, although repulsion reduction is asymmetrical, depleted explants are more repelled than uninjected ones, which is again different from Pak1 requirements in heterotypic explant combinations. It also contrasts with CILa regulation by ephrinB1 in mosaic explants where ephrinB1-depleted cells can extend lamellipodia on normal cells, but not vice versa (Nagel and Winklbauer, 2018). Thus, ephrinB1 is not an effector of Pak1 in the context of LEM explant repulsion on fibronectin, and not acting through controlling CILa.
On CS, homotypic ephrinB1-MO/ephrinB1-MO explant lateral margins are repelled as strongly as those of non-treated explants, although margin retraction on fibronectin is reduced by ephrinB1 depletion (Fig. 4 B,G). As in the case of Pak1, lateral repulsion is independent of ephrinB1 function. Further, front-back fusion is lost. Front-back asymmetry is also diminished except in the ephrinB1-MO front/uninjected back combination, which reproduces the behavior on fibronectin substrate (Fig. 4 F,G). From its explant margin repulsion/fusion behavior, ephrinB1 appears neither on fibronectin nor on CS as if acting downstream of Pak1.
To identify the context of ephrinB1 function, we considered that in the Xenopus gastrula, Dishevelled (Dvl) protein binds via its DEP domain to ephrinB1 to regulate tissue separation (Tanaka et al., 2003; Lee et al., 2006). Expressing the isolated DEP domain, in the form of the DvlDEP+ construct, should interfere with this function of ephrinB1, and indeed, respective explants on CS interacted strikingly similar to ephrinB1 morphant explants: lateral repulsion was retained, and front-back contacts became symmetrically repellent (Fig. 4 B,I). Thus, an ephrinB1-Dvl module known from the interaction of different tissues acts presumably also between explants of the same LEM tissue. Moreover, like Pak1 it promotes on CS tissue fusion, not separation, in front-back encounters. Altogether, although both Pak1 and ephrinB1 control CILa, a cell body-protrusion interaction, their respective roles in explant contact behavior are not dominated by this function. Instead, roles in tissue separation-related processes based on cell body-cell body interaction may be important.
The Wnt receptor Frizzled7 (Xfz7) controls tissue separation at the ectoderm-mesoderm boundary (Winklbauer et al., 2001; Luu et al., 2015), and when examined for explant repulsion on CS, its knockdown profile resembled the respective DN-Pak1 signature (Fig. 4 A,H). This raised the question whether Pak1 acted downstream of Xfz7 to control CILa, or tissue separation, or both. Since directional migration on CS seems to involve either process, we used this assay to identify the non-canonical Xfz7 pathway involved in Pak1 activation. We then showed that Pak1 controls tissue separation in the gastrula, and Xfz7 signaling may thus impact explant repulsion via Pak1.
Non-canonical Xfz7 signaling controls directional LEM migration upstream of Pak1
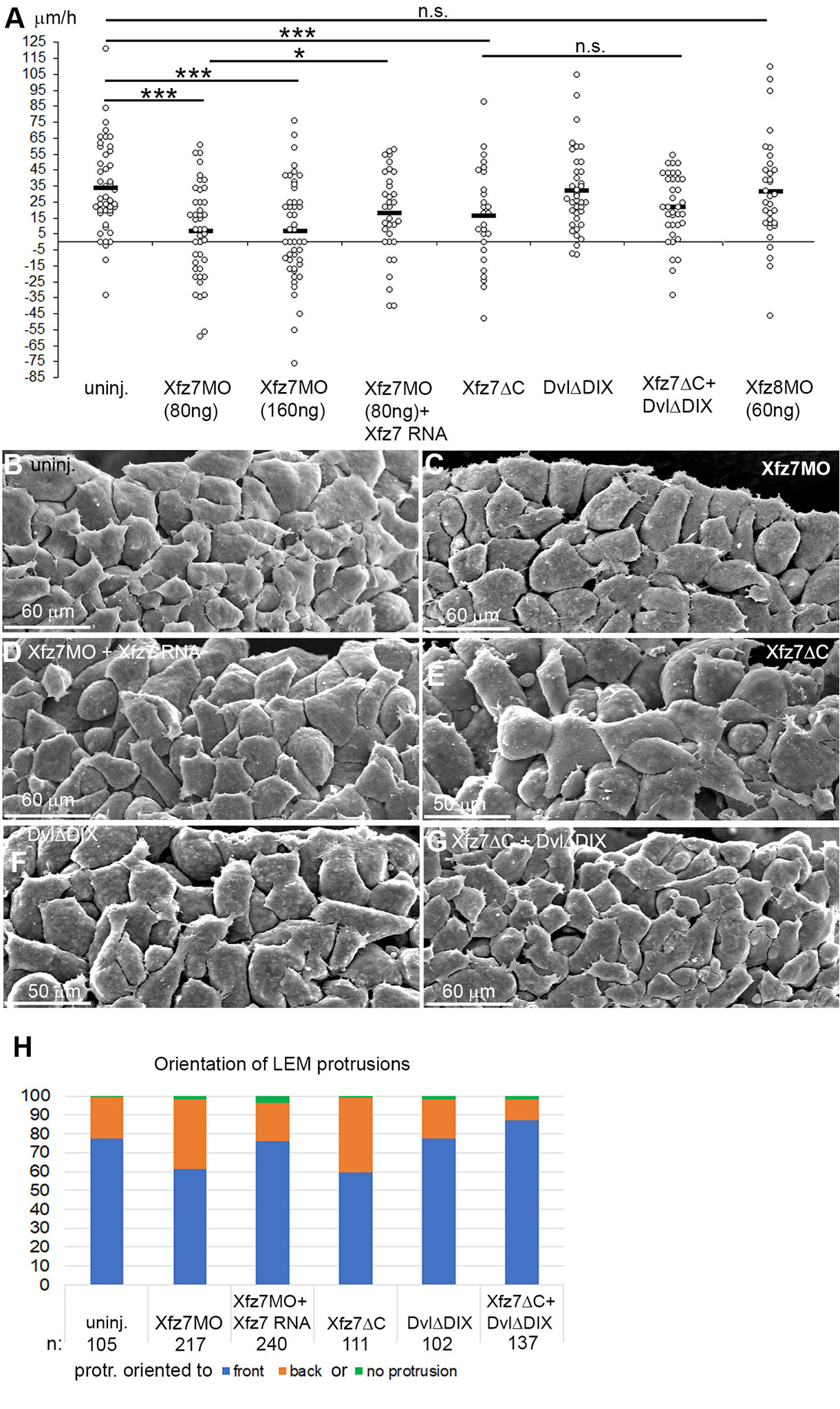
Knockdown of Xfz7 but not Xfz8 strongly reduced, and co-injection of Xfz7 morpholino and Xfz7 mRNA moderately rescued directionality on CS (Fig. 5A). A dominant-negative Xfz7ΔC construct that lacks the cytoplasmic domain (Winklbauer et al., 2001) likewise affected directionality (Fig. 5A). Xfz7 signaling is mediated by Dvl, and a DvlΔDIX construct that inhibits canonical Wnt signaling in Xenopus (Wallingford et al., 2002) did not block directional migration nor affect it downstream of Xfz7ΔC. Effects on directional migration are correlated with altered cell orientation. Normally, protrusions point preferentially towards the animal pole (Fig. 5 B,H). Knockdown of Xfz7 or expression of Xfz7ΔC diminished orientation, but orientation was rescued in morphants by co-injection of Xfz7 mRNA (Fig. 5 C-E, H). As in directional migration, DvlΔDIX had no effect on cell orientation when expressed alone but rescued it downstream of Xfz7ΔC (Fig. 5 F-H). Together, the results indicate that a non-canonical Xfz7 signal is required for directional migration and LEM cell orientation.
Fig. 5. Non-canonical Xfz7 signaling controls directional LEM migration.
(A) Directional migration of LEM explants on conditioned substrate (CS), treated as indicated. Velocities of explants towards the animal pole (positive values) or away (negative values) for each explant (dot) is indicated; black bars, averages; ns, not significant; *, p ≤ 0.05; ***, p ≤ 0.001. (B-G) SEM images of LEM from stage 11 embryos, substrate-apposed side from which the blastocoel roof was removed after fixation. (B) Shingle arrangement of cells, orientation of lamellipodia to the animal pole in non-injected LEM. Xfz7MO injection and (C) Xfz7ΔC expression (E) reduce orientation; Xfz7 mRNA co-injection rescues it (D). DvlΔDIX (F) co-injection with Xfz7ΔC (G) rescues orientation. (H) Percentage of protrusions pointing towards or away from the front edge of the LEM. n, number of cells.
To characterize this non-canonical pathway, we further examined the role of Dvl (Fig. 6A). Co-injection of morpholinos targeting Dvl1 and Dvl2 (Lee et al., 2006; Sheldahl et al., 2003) reduced directionality. Neither DvlΔD1, an inhibitor of the planar cell polarity branch of the pathway (Wallingford et al., 2002), nor DvlΔDEP which activates PCP signaling (De Calisto et al., 2005) significantly inhibited directional migration. On the other hand, DvlDEP+ which inhibits both PCP and Wnt/Ca2+ signaling (Tada and Smith, 2000; Sheldahl et al., 2003) almost completely randomized the direction of migration, and directionality was partially rescued by co-expression of Dvl2. This effect of DvlDEP+ has been putatively linked to an ephrinB1 function in the present context (see Fig. 4). However, another Dvl construct, DvlΔPDZ, which only interferes with the Wnt/Ca++ branch of Wnt signaling (Wallingford and Habas, 2005; Winklbauer et al., 2001) also reduced directionality (Fig. 6A).
Fig. 6. Non-canonical Xfz7 signaling upstream of Pak1 and tissue separation.
(A,B) Directional migration of LEM explants treated as indicated. PTX, pertussis toxin; Chel, chelerythrin. Velocities of explants towards the animal pole (positive values) or away (negative) for each explant is indicated, black bars, averages. Blue bar, average of uninjected control (from Fig. 5A). ns, not significant; *, p ≤ 0.05; ***, p ≤ 0.001. (C-G) Phalloidin staining, LEM explants on fibronectin, treatment indicated. (H) Tissue separation assay, percent of test explants (above bar) remaining on explanted blastocoel roof (below bar), treatments as indicated. n, number of explants.
The Wnt/Ca++ pathway signals through a pertussis toxin (PTX) sensitive trimeric G-protein and activation of PKCα and Cdc42 downstream of Dvl (Winklbauer et al., 2001). Consistent with this, PTX blocked directionality, which was rescued by coexpression of PKCα (Fig. 6B). In addition, inhibition by Xfz7ΔC was reversed by PKCα overexpression, which in turn was sensitive to the PKC inhibitor chelerythrin, administered during migration. This places PKCα downstream of Xfz7 and the PTX-targeted G-protein. A dominant-negative T-Daam1 construct did not abrogate directional migration (Fig. 6B, left), confirming that the pathway differed from the Daam1-dependent branch that controls convergent extension of the chordamesoderm (Habas et al., 2001).
Pak1 and Cdc42 are both required for directional migration (Nagel et al., 2009). We confirmed that dominant-negative Cdc42 inhibits directionality and showed that constitutively active Cdc42 reverses inhibition of directional migration by Xfz7-morpholino (Fig. 6B), consistent with Cdc42 acting downstream of Xfz7. The effect of dominant-negative Cdc42 was rescued by constitutively active Pak1 (Fig. 6B), placing Pak1 downstream of Cdc42. For confirmation, we visualized interior protrusions in LEM explants on fibronectin (Fig. 6 C-G). Expression of dominant-negative Cdc42 or KD-Pak1 induced interior lamellipodia, and constitutively active Pak1 suppressed this effect. Together, our results indicate that a Xfz7 – Dvl – G-protein – PKCα – Cdc42 pathway activates Pak1, which is necessary for directional LEM migration. This expands our previously identified Pak1 – ephrinB1 pathway that promotes CILa, by adding upstream a non-canonical Xfz7 cascade (see Fig. 7A). Pak1-dependent explant repulsion is not explained by its effect on CILa. However, with Pak1 acting downstream of a Xfz7 pathway known to control ectoderm-mesoderm boundary formation (Winklbauer et al., 2001; Luu et al., 2015), we asked whether Pak1 was required for this tissue separation process.
Pak1 controls ectoderm-mesoderm tissue separation
In an in vitro assay for tissue separation (Wacker et al., 2000; Winklbauer et al., 2001), small mesoderm explants stay on the surface of large, explanted ectoderm layers, exhibiting separation behavior, whereas ectodermal test explants reintegrate rapidly into their layer of origin (Fig. 6H). Inhibition of Pak1 in the mesoderm by KD-Pak1 strongly reduced separation behavior, consistent with a role of Xfz7/Pak1 in the mesoderm in tissue separation. With ephrinB1 not acting downstream of Pak1 in explant repulsion, Pak1 may control this process as well as tissue separation directly. Unexpectedly, KD-Pak1 expression in ectoderm similarly reduced tissue separation, and even more surprisingly, blocking Pak1 function in both tissues rescued separation (Fig. 6H). Apparently, ectoderm and mesoderm remain separated when Pak1 is active in both tissues, or in none of the two, but the tissues fuse when it is active in one and not the other. When this interaction logic is applied to LEM explants, it explains their contact behaviors on fibronectin where two non-injected or two KD-Pak1 expressing explants repel each other but heterotypic contacts favor fusion, with an asymmetry that mimics the normal front-back asymmetry on CS. However, interaction with the CS reverses the function of the Xfz7/Pak1 module, and fusion of explants, not repulsion is mediated by what was identified here as a tissue separation factor.
Discussion
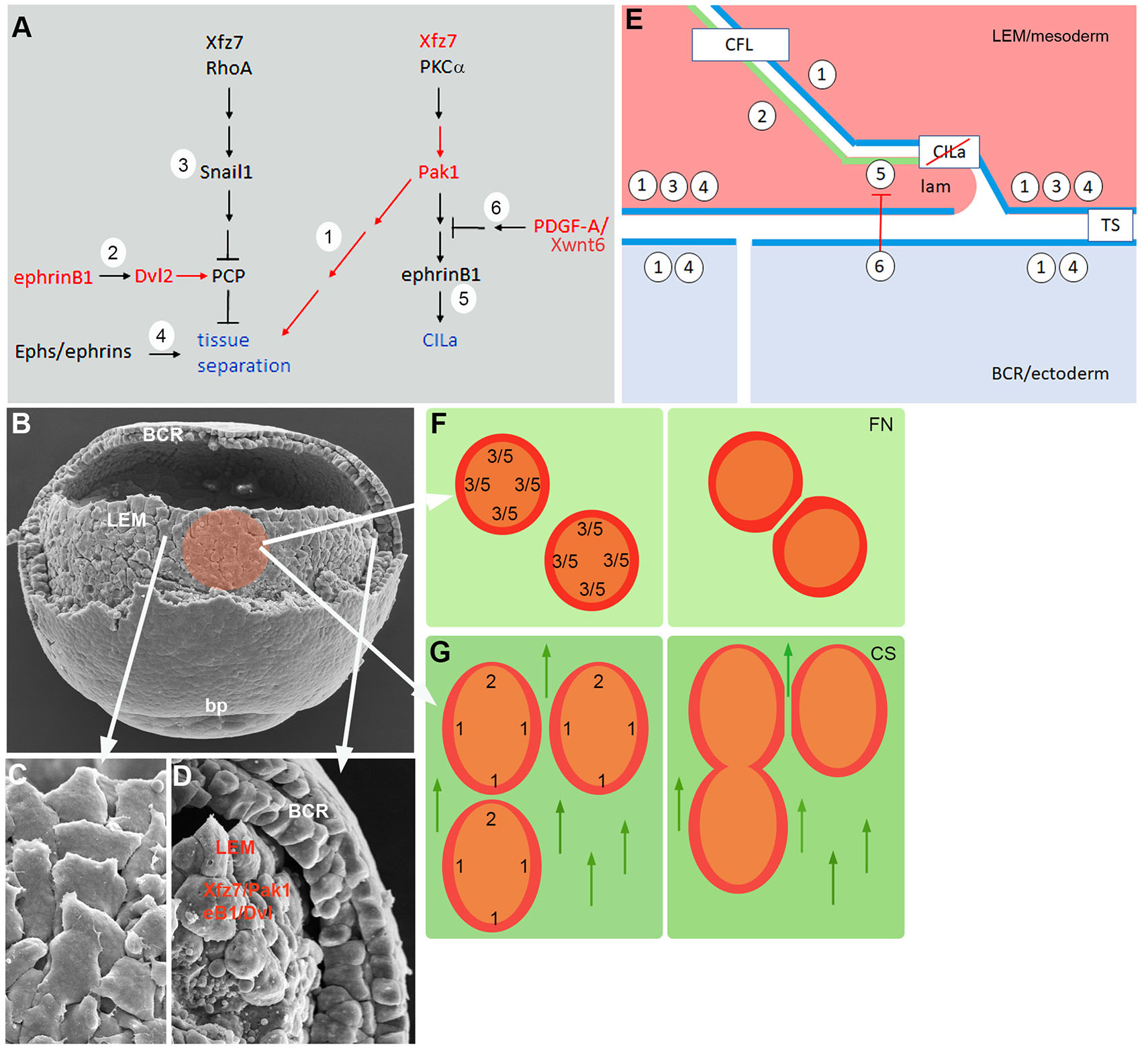
The Xenopus gastrula ectoderm deposits guidance cues in the extracellular matrix that polarize the LEM and direct its movement (Nakatsuji and Johnson, 1983; Winklbauer and Nagel, 1991; Nagel et al., 2004; Nagel and Winklbauer, 2018). We analyzed the responses to contact between explants migrating on CS to further characterize substratum-dependent polarization. We interfered with candidate regulators of explant repulsion/fusion, and the respective front-lateral-back contact response patterns of treated explants revealed three groups of factors: Xfz7/Pak1, ephrinB1/Dvl2, and PDGF-A/Xwnt6. The groups can be associated with signaling pathways known to control CILa and tissue separation (Fig. 7A). Thus, a Xfz7 pathway involving PKCα has been partially identified previously (Winklbauer et al., 2001; Luu et al., 2015). It is linked here to a Pak1-ephrinB1 cascade that controls LEM migration via CILa (Nagel and Winklbauer, 2018) (1,5 in Fig. 7A). A Xwnt6/PDGF-A-dependent signal inhibits this pathway at one side of the cells such that polarization of LEM protrusive activity ensues (Nagel and Winklbauer, 2018) (6 in Fig. 7A). Another Xfz7 pathway acting through RhoA and controlling Snail1 expression interferes with a Dvl- and Pk1-dependent planar cell polarity (PCP) mechanism at the ectoderm-mesoderm boundary to promote tissue separation (Luu et al., 2015) (3 in Fig. 7A). We propose that this PCP function is activated in the LEM by ephrinB1/Dvl2 (Tanaka et al., 2003; Lee et al., 2006) (2 in Fig. 7A). Tissue separation is also controlled by independent Eph/ephrin signaling (4 Fig. 7A) and by Pak1 activated in the Xfz7/PKCα pathway (1 in Fig. 7A). Xwnt6/PDGF-A impacts the Xfz7/PKCα cascade below Pak1, and thus only controls CILa, while inhibition of Pak1 interferes with both CILa and tissue separation.
Fig. 7. LEM contact behaviors.
(A) Signaling pathways controlling tissue separation and contact inhibition of lamellipodia (CILa) (blue). Pathway elements 1 – 6 are described and referenced in the text. Red, factors tested in the present work. (B-D) SEM images of middle gastrula. (B) LEM as seen after partial removal of dorsal blastocoel roof (BCR); bp, blastopore. Colored area, prospective LEM explant. (C) Train of underlapping LEM cells, animally oriented. (D) LEM in sagittally fractured embryo. Separation from BCR depends on ephrinB1 and Dvl2 (eB1/Dvl) and on Xfz7 and Pak1 (Xfz7/Pak1) in the LEM. (E) Model of contact behavior between LEM cells (pink), and tissue separation between LEM and BCR cells (blue). Numbers represent pathway elements defined in (A). Apposition of ephrinB1/Dvl2 dominated (2 in A) front surface (green) and Pak1 dominated (1 in A) surface (blue) of LEM cells leads to adhesion via contact following of locomotion (CFL). At the mesoderm-ectoderm boundary, Pak1 surfaces apposition (blue), Xfz7/Snail signaling in LEM, and Eph/ephrin signaling lead to tissue separation (TS). PDGF-A signal from the ectoderm inhibits CILa in LEM lamellipodia (lam). (F) LEM explants on fibronectin (left), repellent margin prevents fusion (right) based on CILa in the absence of PDGF-A (5, 6 in A,E) and tissue separation-like repulsion (3 in A,E). (G) On CS, CILa is suppressed, and explants are polarized. EphrinB1/Dvl2 activity dominates at the front end (2 in A,E) and Pak1 function elsewhere (1 in A,E) (left). This leads via CFL to front-to-back explant fusion and via Pak1-Pak1 tissue separation to lateral repulsion (right). Arrows point to animal pole.
These signaling cascades regulate a complex interplay of protrusion-cell body and cell body-cell body interactions during LEM migration (Nagel et al., 2021; Huang and Winklbauer, 2018), and in the following we summarize the data in a working model of the system (Fig. 7E). The model relies on the basic observation that factors promoting ectoderm-mesoderm tissue separation are used on CS for the inverse effect, to induce tissue fusion. We propose that this switch from tissue repulsion to fusion can be explained by a distinct cell interaction mechanism, contact following of locomotion (CFL).
CFL refers to the formation of “trains” of front-to-back attached cells during collective migration, based on an interplay of preferential front-back adhesion and lateral separation of cells. It occurs for example during mound formation and slug migration in Dictyostelium (Fujimori et al., 2019) or in migrating cultured mammalian cells (Li and Wang, 2018). In the LEM, trains of 2-5 aligned, uniformly oriented cells are seen in the intact embryo in the scanning electron microscope (Fig. 7 B,C) (Nagel et al., 2004; Nagel and Winklbauer, 2018; Nagel et al., 2021) or in time lapse recordings of explants where translocating trains form spontaneously on non-oriented substratum (Nagel and Winklbauer, 2018). The alignment of interior cells with outward oriented marginal cells in explants also points at CFL.
CFL implies a front-back difference in cells that underlies the observed adhesion preferences. Our results regarding the encounters of heterotypic LEM explants assign Xfz7/Pak1 activity to the back sides of cells (Fig. 7 E,G). Notably, in mammalian cells, front-back adhesion in trains also requires non-canonical Fz signaling (Li and Wang, 2018). At the front end of LEM explants, ephrinB1/Dvl2 activity is required (Fig. 7G), and ephrinB1 protein is enriched in a narrow zone at the front of cells (Nagel and Winklbauer, 2018; Nagel et al., 2021). This suggests a restricted front zone marked by ephrinB1 enrichment and a lateral-back zone, consisting of the remainder of the cell body, where Pak1 is activated (Fig. 7 E,G). Interaction between the two different zones is essential for explant fusion and, by extension, at the cell level for CFL. How the regional pathway activities are translated into preferential “heterotypic” adhesion remains to be elucidated.
Our ectoderm-mesoderm tissue separation results for Pak1 are consistent with Xfz7/Pak1 being active over most of the LEM cell surface. They imply that Pak1 is also active in the ectoderm, normally precluding fusion of the two germ layers (Fig. 7 D,E). As expected from the model, fusion occurs when Pak1 or Xfz7 signaling upstream of it is inhibited in the mesoderm, but also when Pak1 function is blocked in the ectoderm. Another network component, PCP signaling which presumably is activated by ephrinB1/Dvl2, must be attenuated at the tissue interface by Xfz7/Snail1 (Luu et al., 2015) (Fig. 7E). Lastly, an Eph/ephrin signaling complex is necessary for ectoderm-mesoderm separation (Rohani et al., 2011, 2014; Park et al., 2011; Barua and Winklbauer, 2022) (Fig. 7E). How Pak1 function is related to this well-studied mechanism is not known.
Intriguingly, factors identified in the context of cell repulsion during boundary formation could function primarily in CFL front-back adhesion and secondarily contribute to tissue separation, for example by generating a back-back configuration at the ectoderm-mesoderm interface (Fig. 7E). In both contexts, cell body-cell body interactions underlie repulsion or adhesion. In CILa, on the other hand, lamellipodia-cell body interaction is regulated (Fig. 7E). PDGF-A released by the ectoderm controls contact behavior in the mesoderm. It interferes with the Xfz7 signal downstream of Pak1 and allows lamellipodia to extend on the surface of adjacent LEM cells, in a spatially restricted manner such that protrusions and CFL polarity are oriented in parallel. CILa and CFL mutually enforcing each other should stabilize the shingle arrangement of cells during directional migration. The front-back propagation of cadherin-dependent force-induced cell polarity (Weber et al., 2012) should contribute to CFL and further stabilize LEM orientation. Thus, cells would not need to perceive the substratum-linked guidance cues during all times, remaining oriented through cycles of ectoderm-mesoderm separation and attachment (Nagel et al., 2021). Such synergies would also explain why LEM cells do not migrate directionally on CS when single (Winklbauer et al., 1992). On the other hand, force-induced polarization may in vitro drive the counter-productive orientation of lamellipodia at the rear margin of explants. This must be overcome by the guidance cue-dependent CFL mechanism for explant fusion to occur.
The model allows an interpretation of the basic features of LEM explant contact behaviors. On fibronectin, explants spread radially, and in the absence of PDGF-A, marginal cells show CILa all around and probably also front-back polarization. Consequently, explants repel each other (Fig. 7F), although they eventually fuse when prevented from move apart over prolonged times. On CS, on the other hand, CILa is directionally suppressed. Importantly, cells and whole explants are also polarized by the substratum-linked guidance cues with respect to CFL, showing front behavior at one end and lateral-back property everywhere else. Thus, explants fuse at front-back interfaces but remain repellent laterally (Fig. 7G).
Lateral LEM cell neighbors can continuously move past each other (Winklbauer et al., 1993; this article), consistent with low lateral adhesiveness. However, sudden lateral detachment over large contact areas between LEM cells also occurs (Winklbauer et al., 1993; present work), hinting at active repulsion. The spontaneous formation of trains on non-polarized substratum (Fig. 7C) (Nagel and Winklbauer, 2018) suggests that weak lateral adhesion and active repulsion on the one hand, and strong front-back attachment on the other may be mechanistically correlated. Repeated lateral separation events by repulsion and peeling, combined with persistent maintenance of front-back contacts, could be essential for directional collective cell migration in the embryo. As the LEM moves forward on the ectodermal BCR, pulling force is mostly transmitted to the ectoderm through a single row of migrating lead cells at the front of the tissue (Sonavane et al., 2017; Nagel et al., 2021) (Fig. 7D). This requires uninterrupted chains of LEM cells behind, mutually attached through strong front-back adhesion. At the same time, repeated BCR attachment and detachment occurs behind the lead cells in small groups of LEM cell, which affects LEM translocation (Nagel et al., 2021) (Fig. 7D). Lateral flexibility between the chains of tightly linked cells would relieve tensions that build up by such motility fluctuations.
Altogether, our analysis traces the basic outlines of an elaborate contact control complex. However, our working model explains explant behaviors after the various treatments only partially. The list of factors in the signaling network is likely incomplete, and the subcellular localization of the signaling processes identified is not sufficiently defined. Finally, the molecular mechanisms of contact-induced cell repulsion are still incompletely known and those of preferential heterotypic front-back adhesion have not even been studied in this system.
Materials and Methods
Embryos, micromanipulations and injections
Xenopus laevis were bred in-house and kept according to University of Toronto Animal Use Protocol 20011765. Embryos from in vitro fertilized eggs were de-jellied with 2% cysteine in 1/10 Modified Barth's Solution [MBS; 88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 0.82 mM MgSO4, 0.33 mM Ca(NO3)2, 0.41 mM CaCl2, 10 mM Hepes (+NaOH), 1% streptomycin, 1% penicillin (pH 7.4) (pH 8.0)]. Embryos were injected at the four-cell stage in both dorsal blastomeres, or animally in all 4 blastomeres in 4% Ficoll, using a Nanoinject II (Drummond Scientific Company), and cultured at 15°C in 1/10 MBS until gastrula stages. Synthetic mRNAs were transcribed from the following plasmids using mMessage mMachine from Ambion, and injected at amounts indicated in pg/blastomere (Table 1).
Table 1
Plasmids used for in vitro mRNA synthesis
| Plasmid | Polym. | Restr.enz. | RNA (pg) | References |
|---|---|---|---|---|
| pT7TS XPak1 | T7 | Xba1 | 360 | Islam N. et al., 2000 |
| pT7TSXKD-Pak1 | T7 | Xba1 | 300 | Bisson N et al., 2003 |
| pT7TSXCA-Pak1 | T7 | Xba1 | 20 | Bisson N et al., 2003 |
| pCS2+ XPKCa | SP6 | Not1 | 500 | Sheldahl et al., 1999 |
| pSP64-PT ptx | SP6 | EcoR1 | 300 | Winklbauer R. et al., 2001 |
| pCS mbGFP | SP6 | Not1 | 100 | Harland R. (Univ. of California, Berkeley, USA) |
| pCS2 mbRFP | SP6 | Not1 | 100 | gift from A. Bruce (Univ. of Toronto, Canada) |
| pXGHE2XlfPDGF | T7 | Nhe1 | 140 | Mercola et al., 1988 |
| pCS2XPDGFRDN | T7 | Nhe1 | 100 | Ataliotis et al., 1995 |
| pCS2+ Xfz7 | SP6 | Kpn1 | 100 | Medina A., Steinbeisser H., 2000 |
| pCS2+ Xfz7DC | SP6 | Kpn1 | 250 | Medina A., Steinbeisser H., 2000 |
| pCS2 XDvl2 | SP6 | Not1 | 250 | Wallingford et al., 2000 |
| pCS2 XDvlDDIX | SP6 | Not1 | 100 | Djiane et al., 2000 |
| pCS2 XDvlDD1 | SP6 | Not1 | 540 | Sokol S., 1996 |
| pCS2+XDvlDDEP | SP6 | Not1 | 300 | Djiane et al.,2000 |
| PCS2+XDvlDPDZ | SP6 | Not1 | 330 | Wallingford et al., 2000 |
| pCS2+XDvlDEP+ | SP6 | Not1 | 250 | Tada M., 2000 |
| pCS2+ rT-Daam | SP6 | Not1 | 1000 | Liu W. et al., 2008 |
| pCS2+DN-Cdc42 | SP6 | Not1 | 400 | gift from Fagotto, F., CRBM, Montpellier, France. |
| pCS2+CA-Cdc42 | SP6 | Not1 | 30 | gift from Symes, K. Boston University, USA |
| pSP64T Xwnt11 | SP6 | Xba1 | 250 | Ku M., Melton D.A., 1993 |
| pCS2+ Xwnt6 | SP6 | Asp718 | 50 | Lavery D.L., 2008 |
Previously characterized morpholino antisense oligonucleotides (Table 2) were obtained from GeneTools, LCC, and injected at doses previously used (40 ng/blastomere unless otherwise stated).
Table 2
Morpholino antisense oligonucleotides
| Morpholino | Sequence 5' - 3' | References |
|---|---|---|
| XDvl1MO | CGTAGATGATTTTGGTCTCAGCCAT | Lee et al., 2006 |
| XDvl2MO | CTTCTGATCCATTTCCAAAGGCATG | Sheldahl et al., 2003 |
| Xfz7MO | CGAGACTGTAGAGGACATGCTGACT | Luu et al., 2015 |
| XeB1MO | GGAGCCCTTCCATCCGCACAGGTGG | Rohani et al., 2014 |
| Xwnt5aMO | GGTGCAACCAGGGCACAATTACTTT | Schambony A, Wedlich D., 2007 |
| Xwnt6MO | TGGTCTTCAGCGCAATCAAGAGAAG | Nagel M., U. of Toronto, Canada |
Microsurgery
Embryos were staged according to Nieuwkoop and Faber (1967). At gastrula stage 10.5, the vitelline membrane was removed with forceps. Microsurgery with mounted eyelashes was performed in MBS at room temperature under a MZ16F (Leica) stereomicroscope.
Preparation of substrata and migration assay
Tissue culture dishes (35 mm) with a polymer coverslip bottom from Ibidi were coated with bovine plasma fibronectin (Sigma) at 200 ng/ml for 1 h and saturated with 1 mg/ml of bovine serum albumin (BSA) for 30 min. To assay directional migration, substrate was conditioned according to Nagel and Winklbauer (1999). Stage 10 blastocoel roof explants were held against the bottom of Greiner tissue culture dishes for 2 hr. After removal of the BCR, substratum was saturated with 50 mg/ml bovine serum albumin (BSA). Three LEM explants (approximately 200 cells per explant) were positioned on conditioned substratum. Time-lapse movies were made at an interval of 1 picture per minute for 1 h under a Zeiss Axiovert 200M Microscope, using the AxioVision software. Chelerythrine (Calbiochem) was used in a concentration of 6 mM. For image collection, a SP8-nonresonant confocal microscope (Leica) with 40x immersion oil objective and a Zeiss Axiovert 200M microscope (PlanNeofluar 20× and 40× oil objectives) with Leica Application SuiteX software or AxioVision LE64 software were used.
F-actin
F-actin was stained in specimens fixed in 4% paraformaldehyde (20 min, 0.01% Triton-X-100 added after 5 min) with Alexa Fluor 488 phalloidin, Alexa Fluor 647 (Thermo Fisher Scientific) at 1:100 in PBS/BSA for 20 min.
Scanning electron microscopy
Embryos were fixed in 2.5% glutaraldehyde/0.1 M sodium cacodylate overnight at 4°C, post-fixed in osmium tetraoxide and dehydrated in an ethanol/0.1 M cacodylate and ethanol/hexamethyl-disilizane series. Specimens were dried overnight, mounted on SEM stubs using conductive carbon tape (Structure Probe), sputter coated with gold-palladium (60%/40%) for 50 s, and imaged with a Hitachi S-2500 scanning electron microscope.
Separation behavior assay
Small explants of internalized mesoderm placed on an explanted layer of blastocoel roof (BCR), i.e., on their normal substrate, remain on its surface while similar explants taken from the BCR sink into the BCR layer (Fig. 6H) (Wacker et al., 2000). Apparently, there is no physical barrier preventing invasion of the BCR cell layer by cells in contact with it, but cells can nevertheless remain on the surface of the BCR, by expressing what we operationally define as separation behavior. By counting the percentage of small cell aggregates remaining on the BCR surface, this assay can be used for semi-quantitative purposes.
Supplementary Material
Movie 1.
LEM explants migrating on CS. The sequence depicted in Fig. 1B is shown at 1 frame/minute. For details, refer to Fig. 1B.
Movie 2.
Front-back encounter of LEM explants migrating on CS viewed at cell level. The sequence of Fig. 2D is shown at 1 frame/minute. For details, refer to Fig. 2D.
Movie 3.
Lateral encounter of LEM explants migrating on CS, viewed above substratum level. The sequence of Fig. 2G’ is shown at 1 frame/minute. For details refer to Fig.2G’.
Acknowledgements
Funding was provided to R.W. by the Canadian Institutes of Health Research (PJT-15614). We thank Dr. Bojan Macanovic for performing tissue separation experiments as a former undergraduate student in our laboratory.
Abbreviations
AP, animal pole ; BCR, blastocoel roof (ectoderm) ; Cdc42, cell division control protein 42 homolog ; CFL, contact following of locomotion ; CILa, contact inhibition of lamellipodia ; CS, conditioned substratum ; Daam1, dishevelled associated activator of morphogenesis 1 ; Dvl, Dishevelled ; DshΔDIX, dishevelled construct, DIX domain deleted ; DvlDEP+, DEP domain of Dvl ; Eph receptor, ephrin receptor ; KD-Pak1, kinase-dead Pak1 ; LEM, leading edge mesendoderm ; lf-PDGF-A, long form of platelet derived growth factor-A ; MO, morpholino ; Pak1, p21-activated kinase 1 ; PDGFRα, platelet derived growth factor receptor a ; PKCa, protein kinase Ca ; PTX, pertussis toxin ; Xfz7, Xenopus frizzled 7 ; Xwnt6, Xenopus wnt 6 ;Declarations
Conflicts of interest
The authors declare that they have no conflict of interests.
References
Ataliotis P., Symes K., Chou M. M., Ho L., Mercola M. (1995). PDGF signalling is required for gastrulation of Xenopus laevis . Development 121: 3099-3110.
Barua D., Winklbauer R. (2022). Eph/ephrin signaling controls cell contacts and formation of a structurally asymmetrical tissue boundary in the Xenopus gastrula. Developmental Biology 490: 73-85.
Bisson N., Islam N., Poitras L., Jean S., Bresnick A., Moss T. (2003). The catalytic domain of xPAK1 is sufficient to induce myosin II dependent in vivo cell fragmentation independently of other apoptotic events. Developmental Biology 263: 264-281.
Canty L., Zarour E., Kashkooli L., François P., Fagotto F. (2017). Sorting at embryonic boundaries requires high heterotypic interfacial tension. Nature Communications 8: 157.
De Calisto J., Araya C., Marchant L., Riaz C. F., Mayor R. (2005). Essential role of non-canonical Wnt signalling in neural crest migration. Development 132: 2587-2597.
Fujimori T., Nakajima A., Shimada N., Sawai S. (2019). Tissue self-organization based on collective cell migration by contact activation of locomotion and chemotaxis. Proceedings of the National Academy of Sciences 116: 4291-4296.
Djiane A., Riou J.F., Umbhauer M., Boucaut J.C., Shi D.L. (2000). Role of frizzled 7 in the regulation of convergent extension movements during gastrulation in Xenopus laevis . Development 127: 3091-3100.
Gong J., Gaitanos T. N., Luu O., Huang Y., Gaitanos L., Lindner J., Winklbauer R., Klein R. (2019). Gulp1 controls Eph/ephrin trogocytosis and is important for cell rearrangements during development. Journal of Cell Biology 218: 3455-3471.
Habas R., Kato Y., He X. (2001). Wnt/Frizzled Activation of Rho Regulates Vertebrate Gastrulation and Requires a Novel Formin Homology Protein Daam1. Cell 107: 843-854.
Huang Y., Winklbauer R. (2018). Cell migration in the Xenopus gastrula . WIREs Developmental Biology 7: 1-21.
Huang Y., Winklbauer R. (2022). Cell cortex regulation by the planar cell polarity protein Prickle1. Journal of Cell Biology 221: e202008116.
Islam N., Poitras L., Moss T., (2000). The cytoskeletal effector xPAK1 is expressed during both ear and lateral line development in Xenopus. The International journal of developmental biology 44: 245-248.
Ku M., Melton D. A. (1993). Xwnt-11 : a maternally expressed Xenopus wnt gene . Development 119: 1161-1173.
Lavery D. L., Davenport I. R., Turnbull Y. D., Wheeler G. N., Hoppler S. (2008). Wnt6 expression in epidermis and epithelial tissues during Xenopus organogenesis . Developmental Dynamics 237: 768-779.
Lee H.S., Bong Y.S., Moore K. B., Soria K., Moody S. A., Daar I. O. (2006). Dishevelled mediates ephrinB1 signalling in the eye field through the planar cell polarity pathway. Nature Cell Biology 8: 55-63.
Lee H.S., Nishanian T. G., Mood K., Bong Y.S., Daar I. O. (2008). EphrinB1 controls cell–cell junctions through the Par polarity complex. Nature Cell Biology 10: 979-986.
Li D., Wang Y. (2018). Coordination of cell migration mediated by site-dependent cell–cell contact. Proceedings of the National Academy of Sciences 115: 10678-10683.
Liu W., Sato A., Khadka D., Bharti R., Diaz H., Runnels L. W., Habas R. (2008). Mechanism of activation of the Formin protein Daam1. Proceedings of the National Academy of Sciences 105: 210-215.
Luu O., Damm E. W., Parent S. E., Barua D., Smith T. H.L., Wen J. W.H., Lepage S. E., Nagel M., Ibrahim-Gawel H., Huang Y., Bruce A. E.E., Winklbauer R. (2015). PAPC mediates self/non–self-distinction during Snail1-dependent tissue separation. Journal of Cell Biology 208: 839-856.
Medina A., Steinbeisser H. (2000). Interaction of Frizzled 7 and Dishevelled inXenopus. Developmental Dynamics 218: 671-680.
Mercola M., Melton D. A., Stiles C. D. (1988). Platelet-Derived Growth Factor A Chain Is Maternally Encoded in Xenopus Embryos . Science 241: 1223-1225.
Nagel M., Winklbauers R. (1999). Establishment of substratum polarity in the blastocoel roof of the Xenopus embryo . Development 126: 1975-1984.
Nagel M., Tahinci E., Symes K., Winklbauer R. (2004). Guidance of mesoderm cell migration in the Xenopus gastrula requires PDGF signaling . Development 131: 2727-2736.
Nagel M., Luu O., Bisson N., Macanovic B., Moss T., Winklbauer R. (2009). Role of p21-activated kinase in cell polarity and directional mesendoderm migration in the Xenopus gastrula . Developmental Dynamics 238: 1709-1726.
Nagel M., Winklbauer R. (2018). PDGF-A suppresses contact inhibition during directional collective cell migration. Development 145: 1-15.
Nagel M., Barua D., Damm E. W., Kashef J., Hofmann R., Ershov A., Cecilia A., Moosmann J., Baumbach T., Winklbauer R. (2021). Capillarity and active cell movement at mesendoderm translocation in the Xenopus gastrula . Development 148: dev198960.
Nakatsuji N., Johnson K. E. (1983). Conditioning of a culture substratum by the ectodermal layer promotes attachment and oriented locomotion by amphibian gastrula mesodermal cells. Journal of Cell Science 59: 43-60.
Nieuwkoop P., Faber J., (1967). Normal Table of Xenopus laevis (Daudin). North Holland, Amsterdam.
Park E. C., Cho G.S., Kim G.H., Choi S.C., Han J.K. (2011). The involvement of Eph–Ephrin signaling in tissue separation and convergence during Xenopus gastrulation movements. Developmental Biology 350: 441-450.
Rohani N., Canty L., Luu O., Fagotto F., Winklbauer R. (2011). EphrinB/EphB Signaling Controls Embryonic Germ Layer Separation by Contact-Induced Cell Detachment. PLoS Biology 9: e1000597.
Rohani N., Parmeggiani A., Winklbauer R., Fagotto F. (2014). Variable Combinations of Specific Ephrin Ligand/Eph Receptor Pairs Control Embryonic Tissue Separation. PLoS Biology 12: e1001955.
Rozema D., Lasko P., Fagotto F., (2023). Dynamic remodelling of cadherin contacts in embryonic mesenchymal cells during differential cell migration. bioRxiv 2023.03.27.534409.
Schambony A., Wedlich D. (2007). Wnt-5A/Ror2 Regulate Expression of XPAPC through an Alternative Noncanonical Signaling Pathway. Developmental Cell 12: 779-792.
Sheldahl L. C., Park M., Malbon C. C., Moon R. T. (1999). Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in aG-protein-dependent manner. Current Biology 9: 695-S1.
Sheldahl L. C., Slusarski D. C., Pandur P., Miller J. R., Kühl M., Moon R. T. (2003). Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. The Journal of Cell Biology 161: 769-777.
Sokol S. Y. (1996). Analysis of Dishevelled signalling pathways during Xenopus development. Current Biology 6: 1456-1467.
Sonavane P. R., Wang C., Dzamba B., Weber G. F., Periasamy A., DeSimone D. W. (2017). Mechanical and signaling roles for keratin intermediate filaments in the assembly and morphogenesis of mesendoderm tissue at gastrulation. Development 144: 4363-4376.
Tada M., Smith J. C. (2000). Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway . Development 127: 2227-2238.
Tanaka M. (2003). Association of Dishevelled with Eph tyrosine kinase receptor and ephrin mediates cell repulsion. The EMBO Journal 22: 847-858.
Wacker S., Grimm K., Joos T., Winklbauer R. (2000). Development and Control of Tissue Separation at Gastrulation in Xenopus. Developmental Biology 224: 428-439.
Wallingford J. B., Fraser S. E., Harland R. M. (2002). Convergent Extension. Developmental Cell 2: 695-706.
Wallingford J. B., Rowning B. A., Vogeli K. M., Rothbächer U., Fraser S. E., Harland R. M. (2000). Dishevelled controls cell polarity during Xenopus gastrulation. Nature 405: 81-85.
Wallingford J. B., Habas R. (2005). The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development 132: 4421-4436.
Weber G. F., Bjerke M. A., DeSimone D. W. (2012). A Mechanoresponsive Cadherin-Keratin Complex Directs Polarized Protrusive Behavior and Collective Cell Migration. Developmental Cell 22: 104-115.
Wen J. W.H., Winklbauer R. (2017). Ingression-type cell migration drives vegetal endoderm internalisation in the Xenopus gastrula. eLife 6: e27190.
Winklbauer R., Nagel M. (1991). Directional mesoderm cell migration in the Xenopus gastrula. Developmental Biology 148: 573-589.
Winklbauer R., Selchow A., Nagel M., Angres B. (1992). Cell interaction and its role in mesoderm cell migration during Xenopus gastrulation. Developmental Dynamics 195: 290-302.
Winklbauer R., Nagel M., Selchow A., Wacker S., (1996). Mesoderm migration in the Xenopus gastrula. The International journal of developmental biology 40: 305-311.
Winklbauer R., Medina A., Swain R. K., Steinbeisser H. (2001). Frizzled-7 signalling controls tissue separation during Xenopus gastrulation. Nature 413: 856-860.