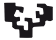Int. J. Dev. Biol. 68: 39 - 45 (2024)
Developmental relationship between junctional epithelium and epithelial rests of Malassez
Open Access | Developmental Expression Pattern | Published: 2 April 2024
Abstract
Keratin 17 (K17) is thought to be a candidate target gene for regulation by Lymphoid Enhancer Factor-1 (Lef-1). K17 is a marker that distinguishes junctional epithelium (JE) from epithelial rests of Malassez (ERM). However, the relationship of Lef-1 to K17 is not clear in this context. Moreover, the expression of other keratins such as K5, K6, K7 and K16 is not reported. Therefore, the aim of our study was to assay the expression of K5, K6, K7, K14, K16, K17 and Lef-1 in postnatal developing teeth, and clarify the corresponding immunophenotypes of the JE and ERM. Upper jaws of Wistar rats aged from postnatal (PN) day 3.5 to PN21 were used and processed for immunohistochemistry. K5 and K14 were intensely expressed in inner enamel epithelium (IEE), reduced enamel epithelium (REE), ERM and JE. There was no staining for K16 in the tissue, except for strong staining in the oral epithelium. Specifically, at PN3.5 and PN7, K17 was initially strongly expressed and then negative in the IEE. At PN16 and PN21, both REE and ERM were strongly stained for K17, whereas K17 was negative in the JE. In addition, K6, K7 and Lef-1 were not detected in any tissue investigated. REE and ERM have an identical keratin expression pattern before eruption, while JE differs from ERM in the expression of K17 after eruption. The expression of K17 does not coincide with that of Lef-1. These data indicate that JE has a unique phenotype different from ERM, which is of odontogenic origin.
Keywords
keratin, lymphoid enhancer factor-1, junctional epithelium, epithelial rests of Malassez, immunohistochemistry
Introduction
Junctional epithelium (JE) is a unique, non-keratinizing tissue which is affixed to tooth on one side and oral sulcular epithelium or connective tissue on the other (Massoth and Dale, 1986). The JE is believed to initially arise from reduced enamel epithelium that fuses with oral epithelium as the tooth erupts into the oral cavity and is replaced, overtime, with a JE derived solely from gingival epithelium (Nishio et al., 2013). Epithelial rests of Malassez (ERM) are developmental residues of Hertwig’s epithelial root sheath, which localize in the periodontal ligament space along the radicular cementum (Beertsen et al., 1997). Since Hertwig’s epithelial root sheath, when first formed, is continuous with the enamel organ, it is entirely possible that the successors to the root sheath and the enamel organ, the ERM and JE, may be continuous as well. Spouge (1984) postulated that these epithelial rests could also contribute to the reactive epithelial lining of the periodontal pocket in periodontitis. However, immunohistochemical analysis by Nishio et al., (2010a; 2010b) revealed that secreted protein odontogenic ameloblast-associated (ODAM) was highly expressed in the JE, while ODAM was not present in ERM from young rodents and was only weakly expressed in ERM from older rodents. These results indicate that JE differs from ERM in the expression pattern of ODAM. Hence, the developmental relationship of JE to ERM remains controversial and many aspects await further investigation.
Keratins are group of intermediate filament proteins that form part of the intracellular cytoskeleton in epithelial cells. These proteins are subdivided into the acidic type Ӏ keratins (K10 to K20) and the neutral-basic type ӀӀ keratins (K1 to K9), and are arranged in heterotypic pairs (Hunter et al., 2001). The expression of keratins is highly cell type specific, making them excellent markers for specific lineage and differentiation (Khanom et al., 2016). A previous study by author (Li et al., 2015) investigated the expression of K14, K17, K19, K10/13 and AE1/AE3 in enamel organ, ERM and JE, with the demonstration that K17 can be used as a marker to distinguish ERM from JE. However, the other members of keratin family are not involved. In addition, evidence has shown that the K17 promoter contains binding sites for transcription factor: lymphoid enhancer factor-1 (Lef-1) (DasGupta and Fuchs, 1999). Lef-1, a protein that belongs to the HMG box family of transcription factors, is known to play a critical role in the epithelial-mesenchymal interactions that underlie appendage morphogenesis (Clevers and Grosschedl, 1996). It has been found that the ectopic expression of Lef-1 in the basal layer of the epidermis leads to K17 synthesis in this compartment (McGowan and Coulombe, 1998), suggesting that K17 may be a Lef-1 response gene. However, it is not clear whether the expression of K17 coincides with that of Lef-1 in the ERM and JE. According to Dias et al., (2005), periodontium development and wound healing share some regulatory mechanisms. Therefore, the aim of the present study was to investigate the expression of K5, K6, K7, K14, K16, K17 and Lef-1 in the enamel organ, ERM and JE, which will expand and extend our previous results, and might provide new insights into the possible roles of the two epithelia in periodontal wound healing or regeneration.
Results
The aim of our study was to determine the expression of Keratin 5 (K5), K6, K7, K14, K16, K17 and Lef-1 in postnatal developing teeth, and clarify the corresponding immunophenotypes of the junctional epithelium (JE) and epithelial rests of Malassez (ERM). To this end, upper jaws of Wistar rats aged from postnatal (PN) day 3.5 to PN21 were dissected and processed for immunohistochemistry.
At PN3.5, the tooth germ reaches the late bell stage. Comprising four distinct layers, namely inner enamel epithelium (IEE), outer enamel epithelium (OEE), stellate reticulum (SR), and stratum intermedium (SI), the enamel organ establishes a connection with the oral epithelium through the dental lamina (DL). Simultaneously, the cervical loop undergoes elongation, transforming into the Hertwig's epithelial root sheath (HERS). Progressing to PN7, the tooth germ transitions into the early crown stage and by PN16, the tooth germ advances to the pre-functional eruption stage, signifying ongoing developmental changes. By PN21, the tooth germ enters the functional eruption stage, accompanied by the juxtaposition of the JE with the enamel space. Notably, at this stage epithelial islands or cords are observed in close proximity to the cementum.
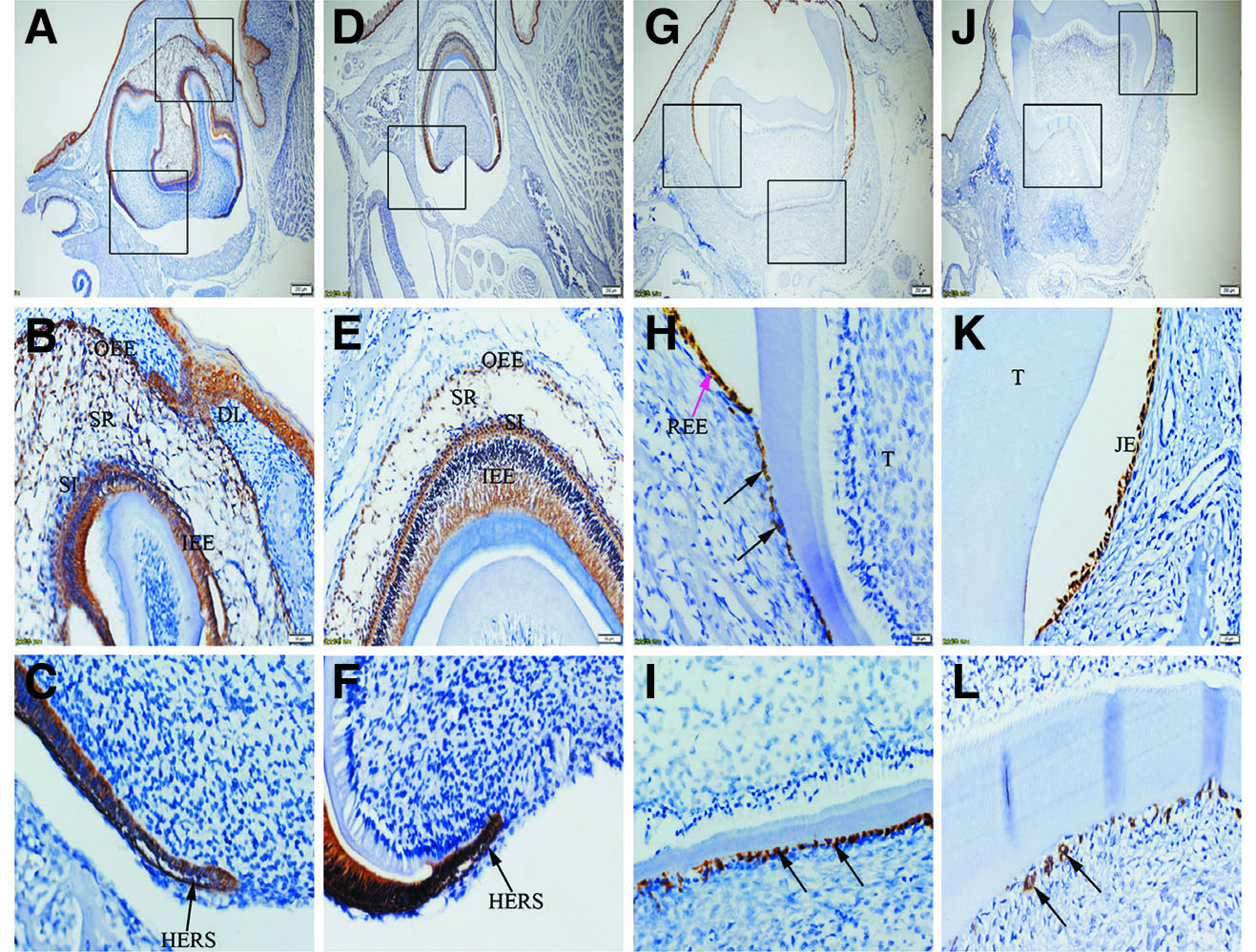
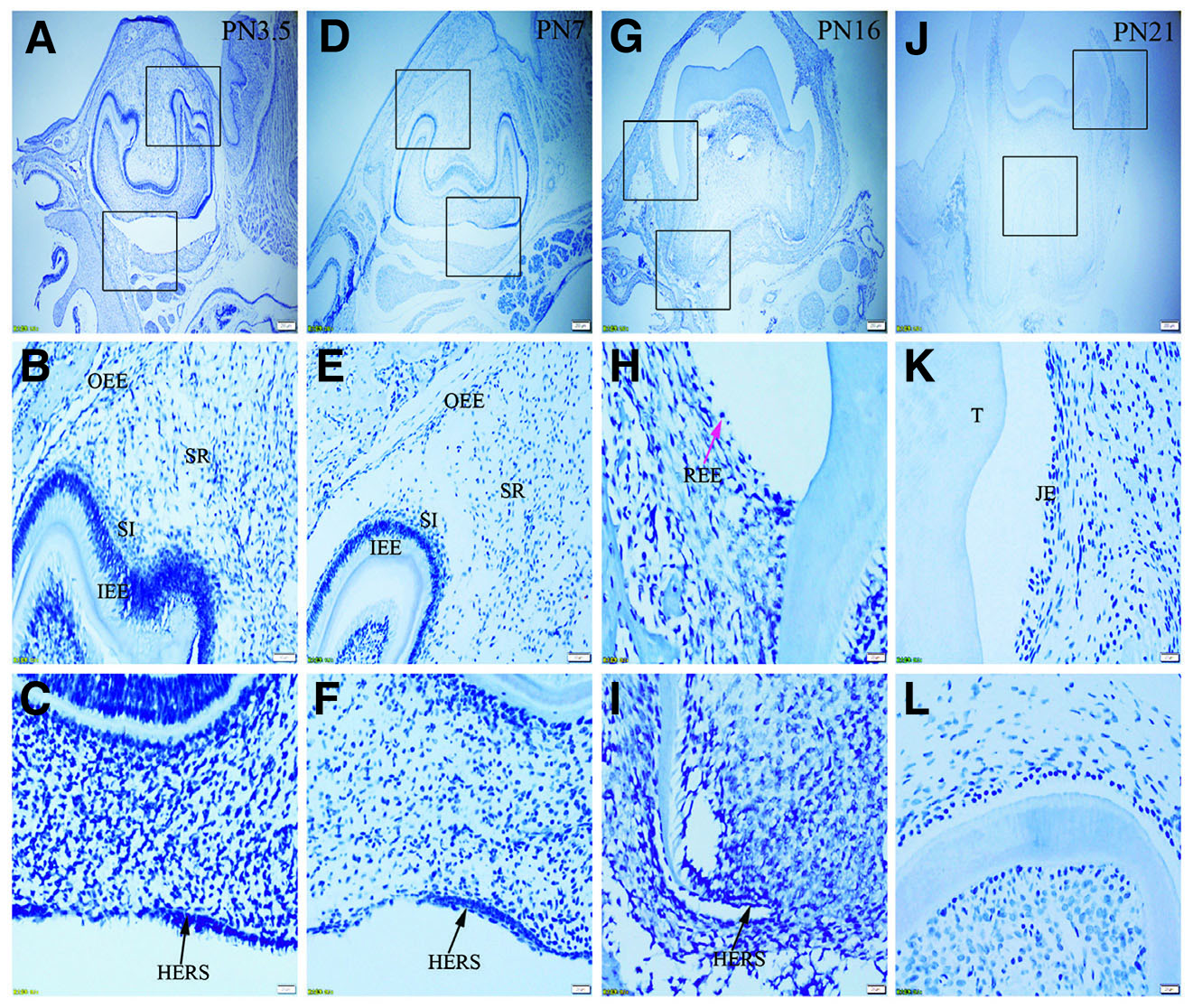
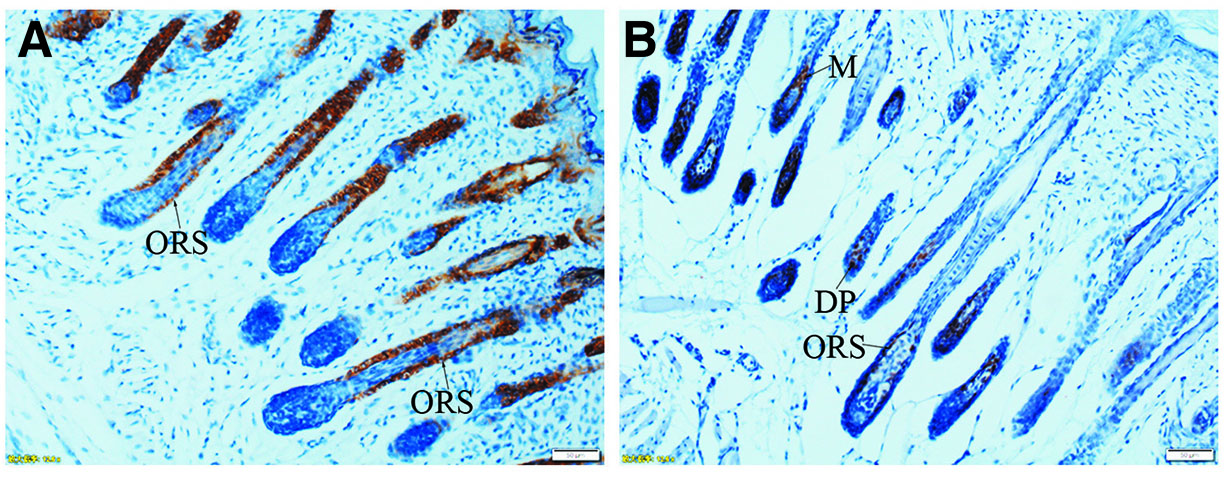
Immunolabelling against K5 and against K14 results in a strong reaction in all tissues analyzed, including OEE, SR, SI, IEE and HERS at all developmental stages (Fig. 1 A-L and Fig. 2 A-L). On the other hand, staining against K6 and K7 did not show any positive structure at the studied timepoints (Fig. 3 A-L, and Fig. 4 A-L). In turn, there was an intense staining for K16 in the oral epithelium, being however this keratin absent in all other tissues (Fig. 5 A-L). In case of K17 we found a unique expression pattern in the postnatal developing tooth: At PN3.5, K17 was strongly expressed in OEE, SI, IEE, SR and HERS (Fig. 6 A-C). Later on, at PN7, strong expression of K17 was retained in OEE, SR, SI and HERS, while the IEE was negative (Fig. 6 D-F). At PN16, REE, ERM and HERS showed strong staining for K17 (Fig. 6 G-I) and, finally at PN21, K17 was strongly expressed in ERM, whereas no staining for K17 was seen in the JE (Fig. 6 J-L). None of the analyzed tissues displayed positive staining for Lef-1 at the studied timepoints (Fig. 7 A-L). Table 1 summarizes the expression of keratins and Lef-1 in the junctional epithelium (JE), ERM/Hertwig epithelial root sheath (HERS) and enamel organ epithelia.
Table 1
Summary of keratins and Lef-1 expressed in the JE, ERM (HERS) and enamel organ epithelia
| Stage (tissues) | K5 | K14 | K6 | K7 | K16 | K17 | Lef-1 |
|---|---|---|---|---|---|---|---|
| Late bell stage (PN3.5) | |||||||
| Stellate reticulum | ++ | ++ | − | − | − | ++ | − |
| Stratum intermedium | ++ | ++ | − | − | − | ++ | − |
| Outer enamel epithelium | ++ | ++ | − | − | − | ++ | − |
| Inner enamel epithelium | ++ | ++ | − | − | − | ++ | − |
| Hertwig epithelial root sheath | ++ | ++ | − | − | − | ++ | − |
| Crown stage (PN7) | |||||||
| Stellate reticulum | ++ | ++ | − | − | − | ++ | − |
| Stratum intermedium | + | ++ | − | − | − | ++ | − |
| Outer enamel epithelium | ++ | ++ | − | − | − | ++ | − |
| Inner enamel epithelium | ++ | ++ | − | − | − | − | − |
| Hertwig epithelial root sheath | ++ | ++ | − | − | − | ++ | − |
| Pre-functional eruption stage (PN16) | |||||||
| Reduced enamel epithelium | ++ | ++ | − | − | − | ++ | − |
| Epithelial rests of Malassez | ++ | ++ | − | − | − | ++ | − |
| Functional eruption stage (PN21) | |||||||
| Junctional epithelium | ++ | ++ | − | − | − | − | − |
| Epithelial rests of Malassez | ++ | ++ | − | − | − | ++ | − |
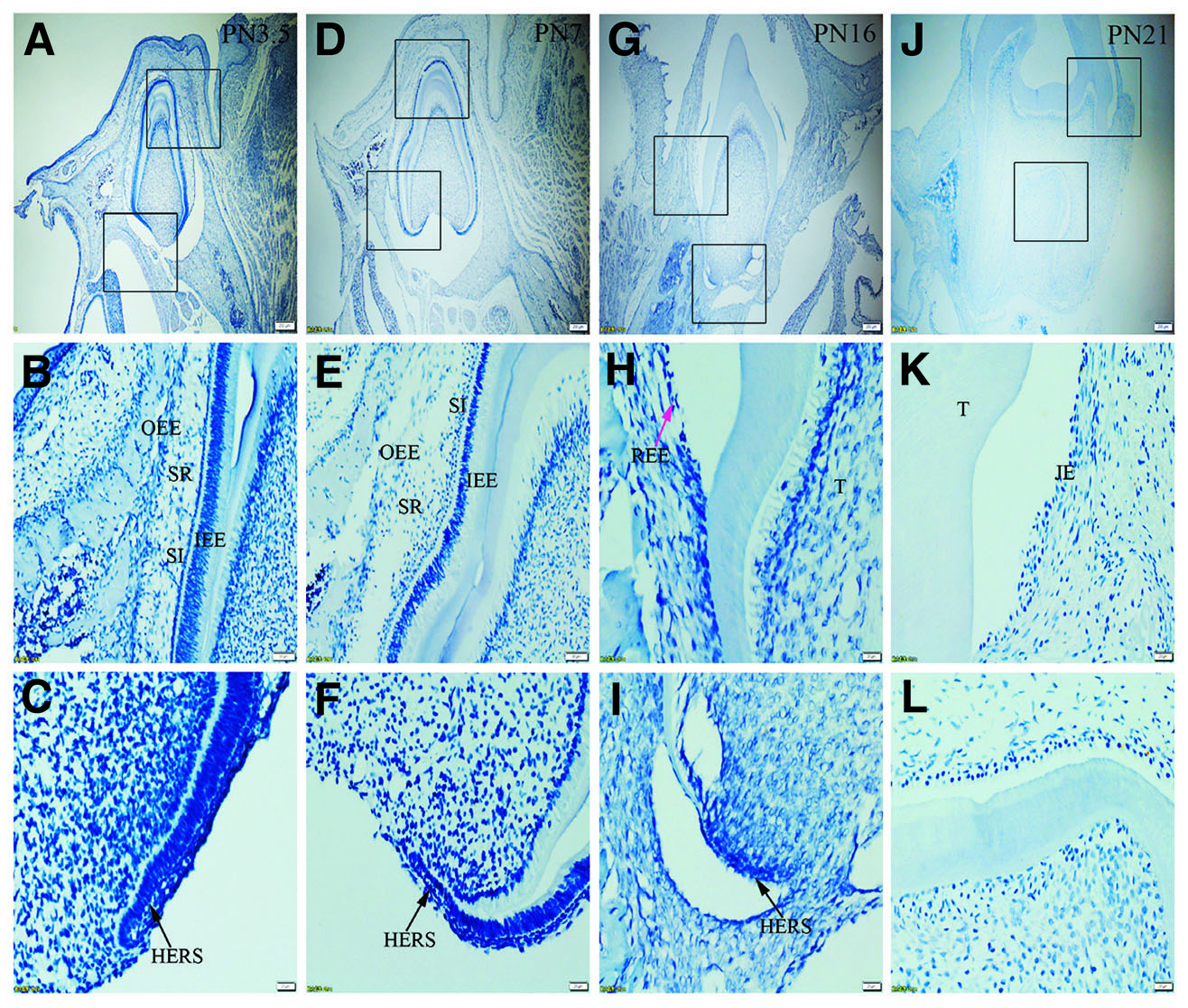
Fig. 1. Expression of K5 in JE, ERM (HERS) and epithelia of the enamel organ.
The boxed regions in A, D, G and J are enlarged in B and C, E and F, H and I, and K and L, respectively. (A-C) At PN3.5, K5 is strongly expressed in outer enamel epithelium (OEE), stellate reticulum (SR), inner enamel epithelium (IEE), stratum intermedium (SI) and Hertwig epithelial root sheath (HERS). (D-F) At PN7, similar to PN3.5, moderate to strong staining for K5 is localized in OEE, SR, SI, IEE and HERS. (G-I) At PN16, K5 is intensely expressed in reduced enamel epithelium (REE) (purple arrow) and epithelial rests of Malassez (ERM) (black arrows). (J-L) At PN21, ERM and junctional epithelium (JE) exhibit strong staining for K5. (A,D,G,J) Scale bar, 100 μm. (B,E) Scale bar, 50 μm. (C,F,H,I,K,L) Scale bar, 20 μm. DL, dental lamina; T, tooth surface.
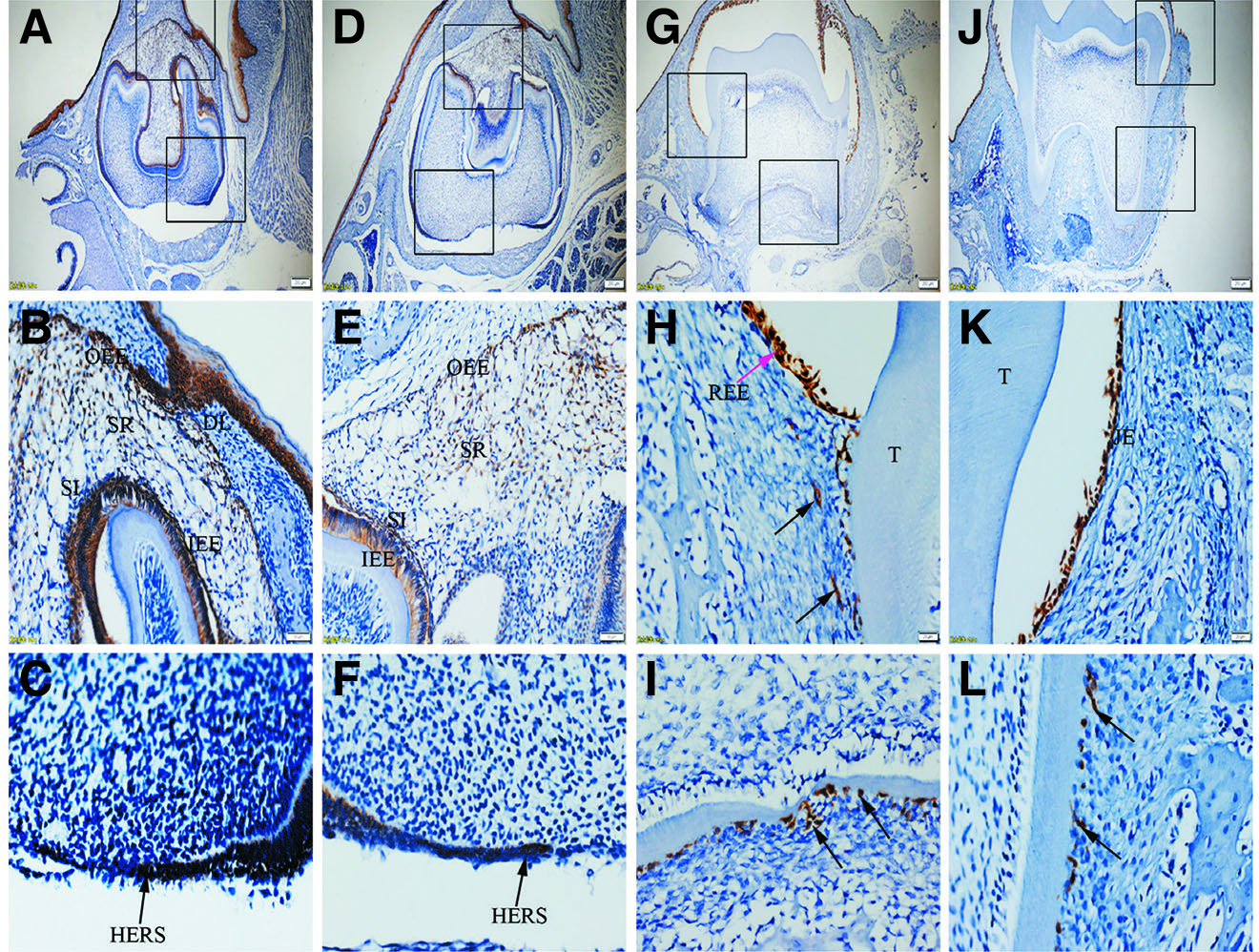
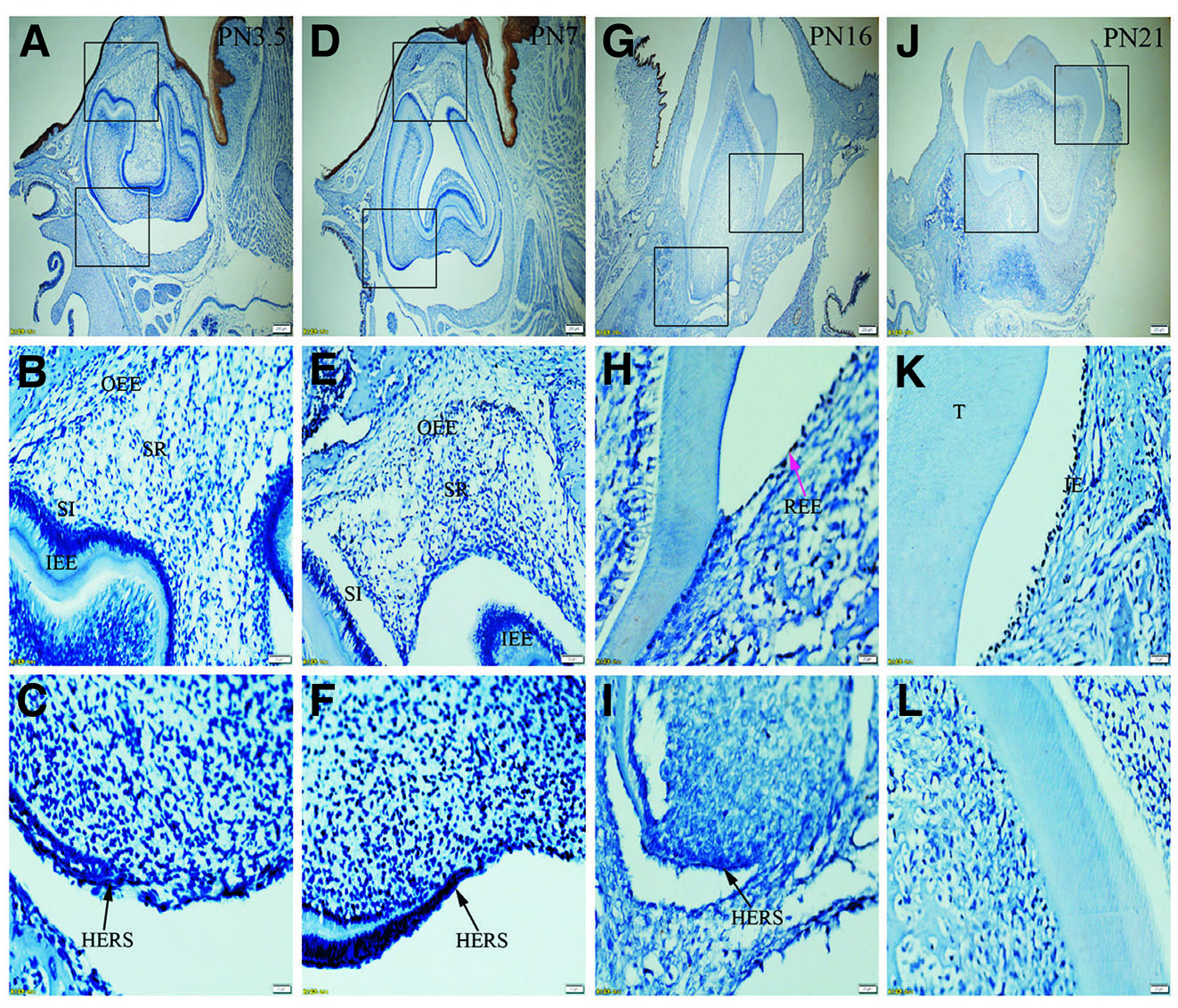
Fig. 2. Expression of K14 in JE, ERM (HERS) and epithelia of the enamel organ.
The boxed regions in A, D, G and J are enlarged in B and C, E and F, H and I, and K and L, respectively. (A-L) Similar to K5, K14 is strongly expressed in SR, OEE, IEE, SI, HERS, REE (purple arrow), ERM (black arrows) and JE. (A,D,G,J) Scale bar, 100 μm. (B,E) Scale bar, 50 μm. (C,F,H,I,K,L) Scale bar, 20 μm. DL, dental lamina; T, tooth surface.
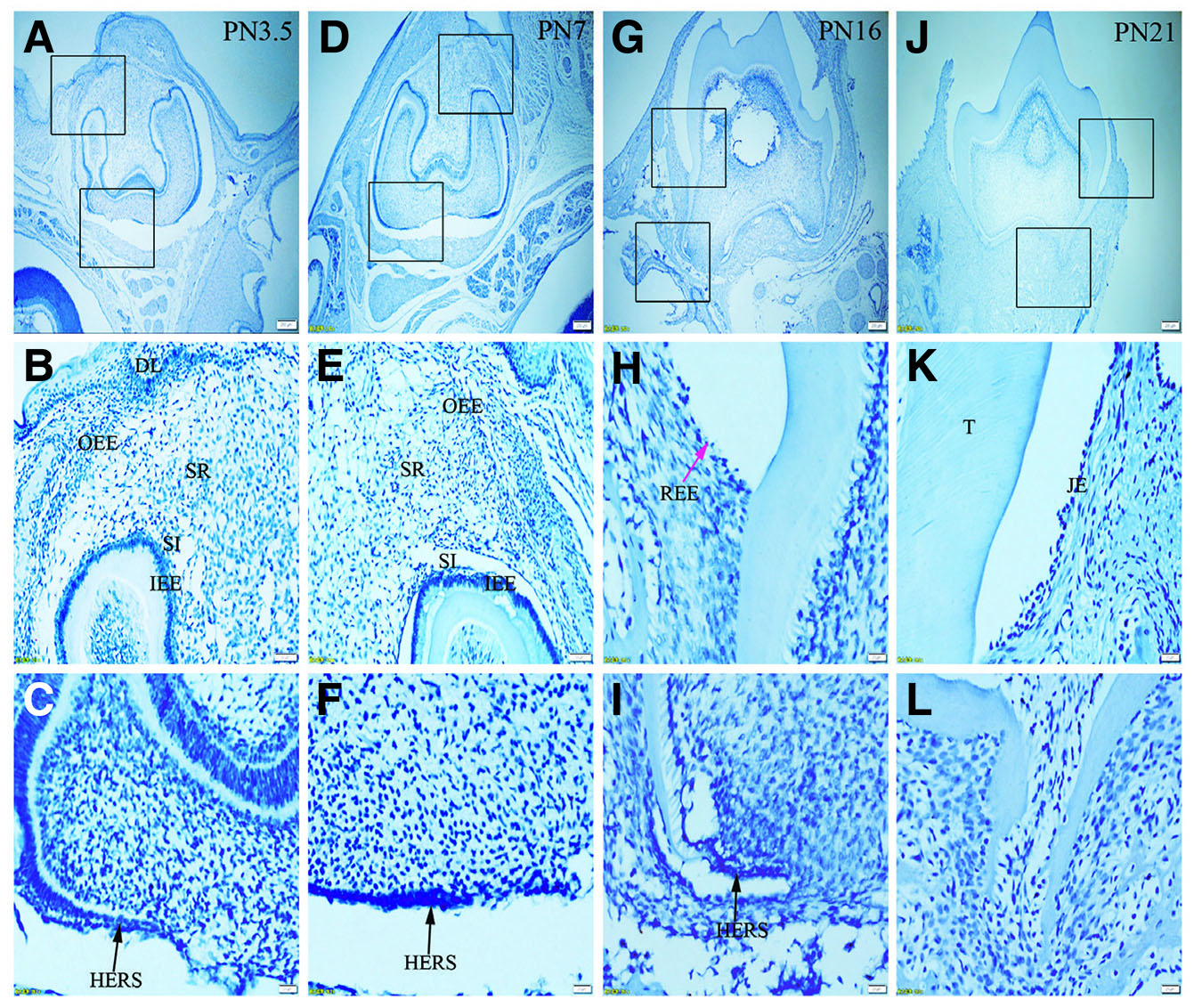
Fig. 3. Expression of K6 in JE, ERM (HERS) and epithelia of the enamel organ.
The boxed regions in A, D, G and J are enlarged in B and C, E and F, H and I, and K and L, respectively. (A-L) There is no positive staining for K6 in any tissue studied. Sections were counterstained with Mayer's hematoxylin solution.(A,D,G,J) Scale bar, 100 μm. (B,E) Scale bar, 50 μm. (C,F,H,I,K,L) Scale bar, 20 μm. T, tooth surface.
Fig. 4. Expression of K7 in JE, ERM (HERS) and epithelia of the enamel organ.
The boxed regions in A, D, G and J are enlarged in B and C, E and F, H and I, and K and L, respectively. (A-L) Negative staining for K7 is seen in the epithelia investigated. (A,D,G,J) Scale bar, 100 μm. (B,E) Scale bar, 50 μm. (C,F,H,I,K,L) Scale bar, 20 μm. T, tooth surface.
Fig. 5. Expression of K16 in JE, ERM (HERS) and epithelia of the enamel organ.
The boxed regions in A, D, G and J are enlarged in B and C, E and F, H and I, and K and L, respectively. (A-L) There is no positive staining in the tissue except that intense reaction with K16 is observed in oral epithelium. (A,D,G,J) Scale bar, 100 μm. (B,E) Scale bar, 50 μm. (C,F,H,I,K,L) Scale bar, 20 μm. T, tooth surface.
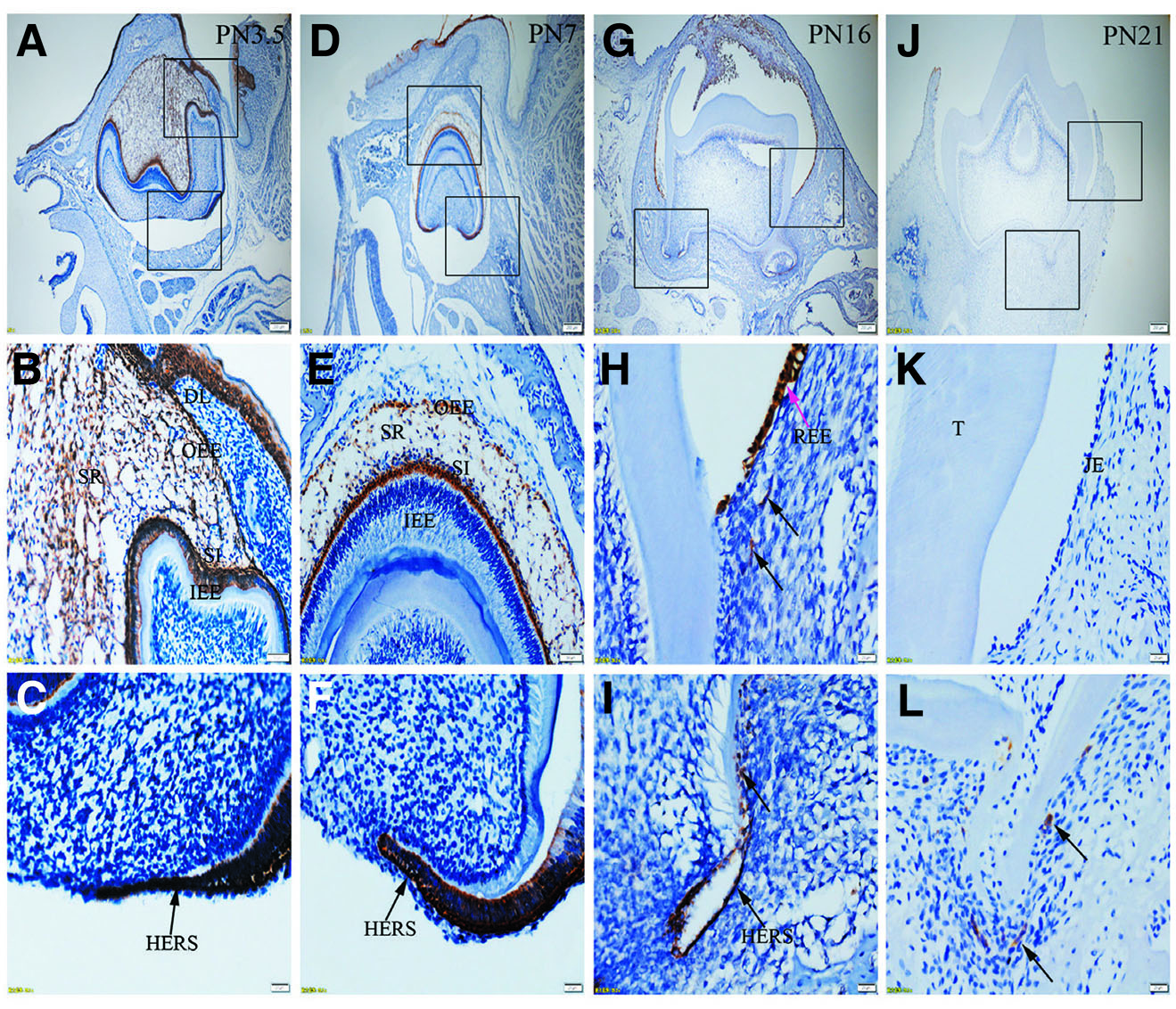
Fig. 6. Expression of K17 in JE, ERM (HERS) and epithelia of the enamel organ.
The boxed regions in A, D, G and J are enlarged in B and C, E and F, H and I, and K and L, respectively. (A-C) At PN3.5, K17 is strongly expressed in SI, IEE, SR, OEE and HERS. (D-F) At PN7, OEE, SR, SI and HERS are strongly stained for K17, while IEE is negative to K17. (G-I) At PN16, Strong staining for K17 is localized in REE (purple arrow) and ERM (black arrows). (J-L) At PN21, K17 is negative in JE but strongly expressed in ERM. (A,D,G,J) Scale bar, 100 μm. (B,E) Scale bar, 50 μm. (C,F,H,I,K,L) Scale bar, 20 μm. DL, dental lamina; T, tooth surface.
Fig. 7. Expression of Lef-1 in JE, ERM (HERS) and epithelia of the enamel organ.
The boxed regions in A, D, G and J are enlarged in B and C, E and F, H and I, and K and L, respectively. (A-L) There is no staining for Lef-1 in the epithelia investigated. Sections were counterstained with Mayer's hematoxylin solution.(A,D,G,J) Scale bar, 100 μm. (B,E) Scale bar, 50 μm. (C,F,H,I,K,L) Scale bar, 20 μm. T, tooth surface.
Discussion
K5 and K14 are the major keratins expressed by basal cells of stratifying epithelium (Mackenzie et al., 1991). Previous studies documented that K5/K14 was widely localized in ERM, JE and enamel organ (Li et al., 2015; Mackenzie et al., 1991; Peters et al., 1995). A study from Alam et al., (2011) has shown that K5 and K14 function to maintain keratinocytes proliferation by increasing Akt phosphorylation, promoting cell cycle progression and inhibiting spontaneous differentiation of keratinocytes. As is known, JE and ERM cells are successfully isolated and cultivated, which provides good reference models for studying the pathogenesis of periodontitis (Matsuyama et al., 1997; Nam et al., 2014). Our results showing the strong staining for K5/K14 in JE and ERM indicate that K5/K14 might play functional roles in maintaining the proliferation of the two epithelial cells. Histological observation demonstrated the absence of ERM in the regenerated periodontal ligament following surgical treatments (Sculean et al., 1998). Recently, ERM is reported to provide a favorable microenvironment for ameliorating the impaired osteogenic potential of human periodontal ligament stem cells (Li et al., 2021). Therefore, in the perspective of periodontal regeneration, it will be interesting to test whether ERM cells overexpressing K5/K14 could be applied to the periodontal treatments.
K7 is a marker of simple epithelium, while K6 and K16 are characteristics of high turnover or migrating epithelium (Hunter et al., 2001; Feghali-Assaly et al., 1994). Peters et al., (1995) confirmed that ERM from human periodontal ligament section was strongly stained for K7, whereas Feghali-Assaly et al., (1994) demonstrated that K7 was absent from ERM. In view of this, K7 expression remain controversial among different studies. In addition, K7 was found to be negative in JE (Mackenzie et al., 1991; Bampton et al., 1991; Pritlove-Carson et al., 1997). In our study, no positive staining for K7 was seen in the epithelia of enamel organ, REE, ERM and JE. Discrepancies in the K7 expression may be due to differences in the species as well as in the affinity, avidity, or epitope availability of the antibodies used. Previous studies noted that K16 was negative in enamel organ and ERM (Mackenzie et al., 1991; Domingues et al., 2000). Moreover, immunohistochemical analysis of human gingiva revealed that K6 and K16 were not expressed in JE (Locke et al., 2008). In accord with these data, the current study confirmed that both K6 and K16 were not present in enamel organ, ERM and JE. As mentioned above, K6 and K16 are characteristic of migrating epithelium, our results showing the negative staining for K6 and K16 in JE might be associated with the normal stability of the gingival sulcus.
The relationship between K17 and Lef-1 was analyzed in the context of postnatal tooth development. Before eruption, K17 was strongly expressed in REE and ERM. After eruption, K17 was negative in JE but strongly expressed in ERM. These results again reinforce the viewpoint that K17 can be used as a marker to distinguish JE from ERM (Li et al., 2015). Additionally, gene expression analysis revealed that Lef-1 is present in the cervical loop of enamel organ (Sasaki et al., 2005). In contrast, our study showed Lef-1 protein was negative in HERS or ERM. The discrepancy between gene and protein expression may be due to the post-transcriptional modification or post-translational processing. Our results that the expression of K17 does not overlap with that of Lef-1 in ERM indicate K17 expression is independent of regulation by Lef-1. Intriguingly, here, similar to K17, Lef-1 was also negative in JE. In order to clarify the relationship between Lef-1 and K17, transgenic or knockout models should be employed to assess whether Lef-1 can influence K17 expression during the formation of JE.
Following surgical treatment of adult periodontitis, the periodontal defect is mainly colonized by gingival epithelial cells with the greatest growth potential. This leads to the formation of a non-physiological, long JE inhibiting the regeneration of periodontal attachment (Gräber et al., 1999; Kantarci, 2022). Data has shown that, in addition to providing mechanical support to tissue architecture, keratin filaments also form complex signaling platforms and interact with various proteins, such as kinases, adaptors and receptors, and thus modulate different signaling pathways associated with various cellular processes, such as protein synthesis, cell growth, and cell differentiation (Kim et al., 2006; Koch and Roop, 2004; Oshima, 2002). Recent studies have revealed that K6, K16 and K17, which are normally not expressed in the interfollicular epidermis, have been recognized as barrier molecules that are rapidly induced in keratinocytes upon wounding. The upregulated keratins alter proliferation, adhesion and migration of keratinocytes (McGowan and Coulombe, 1998; Zhang et al., 2019; Paladini et al., 1996). It is suggested that many of the molecular processes instructing periodontal morphogenesis appear to be mirrored by the processes involved in wound healing (Reed and Diekwisch, 2015). Therefore, elucidation of K6/K16/K17 expression pattern will help us to design a novel approach to regulate the apical growth of gingival epithelial cells and deepen our understanding of biology-based treatment strategies for periodontal regeneration.
In conclusion, before eruption, REE and ERM have identical keratin expression patterns, suggesting that ERM is of odontogenic origin. After eruption, JE differs from ERM in the expression of K17, suggesting JE has a unique phenotype. The expression of K17 does not coincide with that of Lef-1 during postnatal tooth development. Further studies involving functional experiments will be required to investigate the effects of keratins on ERM and JE cells and to dissect the possible roles of the two epithelia in periodontal wound healing and regeneration.
Materials and Methods
Laboratory animals and tissue preparation
Wistar-strain male rats on postnatal (PN) day 3.5, 7, 16, and 21 were used in the present study. Each age group comprised 5 animals. All experimental procedures were approved by the Institutional Committee of Animal Care and Use at Youjiang Medical University for Nationalities. On the specified days, the rats were anaesthetized with chloral hydrate and perfused through the heart first with saline and subsequently with phosphate-buffered formalin solution (10%, pH 7.2, 4°C). Maxillas were dissected and immersed in the same fixative at 4°C overnight. The specimens were then decalcified in 10% EDTA solution (pH 7.4) for 3-8 weeks, after which they were routinely embedded in paraffin. 5 um-thick serial sections were cut in the bucco-lingual plane of the tooth, mounted on poly-L-lysine coated glass slides, and processed for immunohistochemistry.
Immunohistochemistry
Monoclonal antibodies against K5 (ab64081), K14 (ab119695), K6 (ab93279), K7 (ab181598), K16 (ab76416), K17 (ab109725) and Lef-1 (ab137872) (Abcam, Cambridge, UK) were used in the present study. As reported previously (Li and Li, 2023), a standard immunohistochemical procedure was followed using slide-mounted sections. Tissue sections were deparaffinized with xylene and ethanol, then were treated with citric acid (10mM, pH 6.0) (Zhongshan, Beijing, China) to unmask antigen. Endogenous peroxidase was blocked by incubation for 30 min in 0.3% hydrogen peroxidase solution (Zhongshan). Sections were rinsed in phosphate-buffered saline (PBS) and incubated in 5% normal goat serum for 30 min at room temperature. Subsequently, the sections were incubated overnight at 4°C with the primary antibodies (K5, 1:100; K14, 1:100; K6, 1:50; K7, 1:100; K16, 1:100; K17, 1:50; Lef-1, 1:100). After rinsing with PBS, the sections were treated with polyperoxidase-conjugated anti-(mouse/rabbit/goat) IgG for 20 min (Zhongshan). The color reaction was developed using a freshly prepared solution of 3,3-diaminobenzidine tetrahydrochloride (Zhongshan), followed by several washings with distilled water. Finally, the sections were counterstained with Mayer’s hematoxylin solution. Negative control for immunoreaction specificity were made by substituting the primary or secondary antibody with PBS. The intensity of the staining was scored by oral pathologists who did not participate in the present study. Detailed criteria were the followings: −, negative; +/−, weakly positive; +, moderately positive; ++, strongly positive.
Declarations
Conflicts of interest
The authors declare no conflict of interest.
Competing interests
The authors declare that no competing interests exist.
Author contributions
Shubo Li: Design, Methodology, Investigation, Data curation, Formal analysis, Writing-Original draft preparation, Writing-Reviewing and Editing, Resources, Funding acquisition. Shufang Li: Data curation. Mingguo Cao: Data curation, Formal analysis, Writing-Reviewing and Editing.
Funding
This study was supported by Guangxi Natural Science Foundation (grant No: 2020GXNSFAA297058).
References
Alam H., Sehgal L., Kundu S. T., Dalal S. N., Vaidya M. M. (2011). Novel function of keratins 5 and 14 in proliferation and differentiation of stratified epithelial cells. Molecular Biology of the Cell 22: 4068-4078.
Bampton J. L. M., Shirlaw P. J., Topley S., Weller P., Wilton J. M. (1991). Human Junctional Epithelium: Demonstration of a New Marker, Its Growth In Vitro and Characterization by Lectin Reactivity and Keratin Expression. Journal of Investigative Dermatology 96: 708-717.
Beertsen W., McCulloch C. A. G., Sodek J. (1997). The periodontal ligament: a unique, multifunctional connective tissue. Periodontology 2000 13: 20-40.
Clevers H. C., Grosschedl R. (1996). Transcriptional control of lymphoid development: lessons from gene targeting. Immunology Today 17: 336-343.
DasGupta R., Fuchs E. (1999). Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126: 4557-4568.
Dias R. A., Shibata S., Hashimoto‐Uoshima M., Podyma‐Inoue K. A., Ishikawa I., Yanagishita M. (2005). Syndecan‐1 Expression During the Formation of Junctional Epithelium. Journal of Periodontology 76: 696-704.
Domingues M. G., Jaege M. M. M., Araújo V. C., Araújo N. S. (2000). Expression of cytokeratins in human enamel organ. European Journal of Oral Sciences 108: 43-47.
Feghali-Assaly M., Sawaf M.H., Serres G., Forest N., Ouhayoun J. P. (1994). Cytokeratin profile of the junctional epithelium in partially erupted teeth. Journal of Periodontal Research 29: 185-195.
Gräber H.G., Conrads G., Wilharm J., Lampert F. (1999). Role of Interactions Between Integrins and Extracellular Matrix Components in Healthy Epithelial Tissue and Establishment of a Long Junctional Epithelium During Periodontal Wound Healing: A Review. Journal of Periodontology 70: 1511-1522.
Hunter N., Nicholls B., Srivastava M., Chapple C. C., Zoellner H. F., Gibbins J. R. (2001). Reactive pocket epithelium in untreated chronic periodontal disease: possible derivation from developmental remnants of the enamel organ and root sheath. Journal of Oral Pathology & Medicine 30: 178-186.
Kantarci A. (2022). Biological Basis of Periodontal Regeneration. Dental Clinics of North America 66: 1-9.
Khanom R., Nguyen C. T. K., Kayamori K., Zhao X., Morita K., Miki Y., Katsube K., Yamaguchi A., Sakamoto K. (2016). Keratin 17 Is Induced in Oral Cancer and Facilitates Tumor Growth. PLOS ONE 11: e0161163.
Kim S., Wong P., Coulombe P. A. (2006). A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature 441: 362-365.
Koch P. J., Roop D. R. (2004). The Role of Keratins in Epidermal Development and Homeostasis—Going Beyond the Obvious. Journal of Investigative Dermatology 123: x-xi.
Li S., Ge S., Yang P. (2015). Expression of cytokeratins in enamel organ, junctional epithelium and epithelial cell rests of Malassez. Journal of Periodontal Research 50: 846-854.
Li S., Li S. (2023). Temperal and spatial expression of CCN1, CCN3, CCN4, CCN5 and CCN6 proteins in the developing postnatal teeth. Journal of Cell Communication and Signaling 17: 275-285.
Li Y., Liu A., Zhang L., Wang Z., Hui N., Zhai Q., Zhang L., Jin Z., Jin F. (2021). Epithelial Cell Rests of Malassez Provide a Favorable Microenvironment for Ameliorating the Impaired Osteogenic Potential of Human Periodontal Ligament Stem Cells. Frontiers in Physiology 12: 735234.
Locke M., Hyland P. L., Irwin C. R., Mackenzie I. C. (2008). Modulation of gingival epithelial phenotypes by interactions with regionally defined populations of fibroblasts. Journal of Periodontal Research 43: 279-289.
Mackenzie I. C., Rittman G., Gao Z., Leigh I., Lane E. B. (1991). Patterns of cytokeratin expression in human gingival epithelia. Journal of Periodontal Research 26: 468-478.
Massoth D. L., Dale B. A. (1986). Immunohistochemical Study of Structural Proteins in Developing Junctional Epithelium. Journal of Periodontology 57: 756-763.
Matsuyama T., Izumi Y., Sueda T. (1997). Culture and Characterization of Human Junctional Epithelial Cells. Journal of Periodontology 68: 229-239.
McGowan K. M., Coulombe P. A. (1998). Onset of Keratin 17 Expression Coincides with the Definition of Major Epithelial Lineages during Skin Development. The Journal of Cell Biology 143: 469-486.
Nam H., Kim J.H., Kim J.W., Seo B.M., Park J.C., Kim J.W., Lee G. (2014). Establishment of Hertwig’s Epithelial Root Sheath/Epithelial Rests of Malassez Cell Line from Human Periodontium. Molecules and Cells 37: 562-567.
Nishio C., Wazen R., Kuroda S., Moffatt P., Nanci A. (2010a). Disruption of periodontal integrity induces expression of apin by epithelial cell rests of Malassez. Journal of Periodontal Research 45: 709-713.
Nishio C., Wazen R., Kuroda S., Moffatt P., Nanci A. (2010b). Expression pattern of odontogenic ameloblast-associated and amelotin during formation and regeneration of the junctional epithelium. European Cells and Materials 20: 393-402.
Nishio C., Wazen R., Moffatt P., Nanci A. (2013). Expression of odontogenic ameloblast‐associated and amelotin proteins in the junctional epithelium. Periodontology 2000 63: 59-66.
Oshima R. G. (2002). Apoptosis and keratin intermediate filaments. Cell Death & Differentiation 9: 486-492.
Paladini R. D., Takahashi K., Bravo N. S., Coulombe P. A. (1996). Onset of re-epithelialization after skin injury correlates with a reorganization of keratin filaments in wound edge keratinocytes: defining a potential role for keratin 16.. The Journal of cell biology 132: 381-397.
Peters B. H., Peters J.M., Kuhn C., Zöller J., Franke W. W. (1995). Maintenance of cell-type-specific cytoskeletal character in epithelial cells out of epithelial context: Cytokeratins and other cytoskeletal proteins in the rests of Malassez of the periodontal ligament. Differentiation 59: 113-126.
Pritlove-Carson S., Charlesworth S., Morgan P.R., Palmer R.M. (1997). Cytokeratin phenotypes at the dento‐gingival junction in relative health and inflammation, in smokers and non‐smokers. Oral Diseases 3: 19-24.
Reed D. A., Diekwisch T. G., (2015). Biological Aspects of Periodontal Disease. In Stem Cell Biology and Tissue Engineering in Dental Sciences. (Ed. Vishwakarma Ajaykumar , Shi Songtao , Sharpe Paul, Ramalingam Murugan, ) Elsevier, Amsterdam.
Sasaki T., Ito Y., Xu X., Han J., Bringas P., Maeda T., Slavkin H. C., Grosschedl R., Chai Y. (2005). LEF1 is a critical epithelial survival factor during tooth morphogenesis. Developmental Biology 278: 130-143.
Sculean A., Lioubavina N., Theitade J., Karring T. (1998). Absence of Malassez epithelial rests in the regenerated periodontal ligament. A pilot study in the monkey. Journal of Periodontal Research 33: 310-314.
Spouge J. D. (1984). The rests of Malassez and chronic marginal periodontitis. Journal of Clinical Periodontology 11: 340-347.
Zhang X., Yin M., Zhang L. (2019). Keratin 6, 16 and 17—Critical Barrier Alarmin Molecules in Skin Wounds and Psoriasis. Cells 8: 807.