Int. J. Dev. Biol. 68: 19 - 24 (2024)
Disrupted odontoblast differentiation and dentin dysplasia in Epiprofin-deficient mice
Open Access | Original Article | Published: 27 March 2024
Abstract
Tooth formation is a process tightly regulated by reciprocal interactions between epithelial and mesenchymal tissues. These epithelial-mesenchyme interactions regulate the expression of target genes via transcription factors. Among the regulatory elements governing this process, Epiprofin/Sp6 is a zinc finger transcription factor which is expressed in the embryonic dental epithelium and in differentiating pre-odontoblasts. Epiprofin knockout (Epfn-/-) mice present severe dental abnormalities, such as supernumerary teeth and enamel hypoplasia. Here, we describe dentin defects in molars and incisors of Epfn-/- mice. We observed that in the absence of Epfn, markers of early odontoblast differentiation, such as alkaline phosphatase activity, Dsp/Dpp expression, and Collagen Type I deposition, are downregulated. In addition, the expression of tight and gap junction proteins was severely impaired in the predontoblastic cell layer of developing Epfn-/- molars. Altogether, our data shows that Epfn is crucial for the proper differentiation of dental mesenchymal cells towards functional odontoblasts and subsequent dentin-matrix deposition.
Keywords
epiprofin, tooth development, dentin, odontoblast, differentiation
Introduction
Tooth is an organ of ectodermal origin that develops through continuous epithelial-mesenchymal interactions including the initiation, morphogenesis and differentiation stages (Jiménez-Rojo et al., 2010; Thesleff and Hurmerinta, 1981). In mouse molars, at around embryonic day 18.5 (E18.5), cells from the mesenchymal dental papilla (pre-odontoblasts) adjacent to the inner enamel epithelium polarize and differentiate into dentin secreting odontoblasts. The first differentiated odontoblasts appear in the dental papilla, in the central region of the dental cusps. From this moment on, differentiation gradients originate and progressively move towards the base of the cusps (Ruch et al., 1995). Thus, the dentin secreted until the completion of root formation is known as primary dentin and comprises the main bulk of the circumpulpal dentin matrix (Goldberg and Smith, 2004; Smith et al., 1995). It presents inter- and peri-tubular dentin matrices permeated by dentinal tubules containing the odontoblast processes as these cells retreat in a pulpal direction (Goldberg and Smith, 2004).
After odontogenesis is completed, odontoblasts respond to stimuli such as mechanical stress and injury by re-activating dentin secretion, which ultimately leads to the deposition of reactionary dentin. In case of severe damages odontoblasts may die and be renewed by odontoblast-like cells derived from dental mesenchymal stem cells (Goldberg and Smith, 2004; Smith et al., 1995), which secrete the so-called reparative dentin. Both, reactionary and reparative dentin are referred to as tertiary dentin that is usually deposited at the dentin-pulp interface (Goldberg and Smith, 2004). In some occasions, an abnormal matrix of osteodentin can be found in pathological dentin caused by genetic diseases, drug-induced diseases or in response to caries. In this case, osteocyte-like cells become entrapped within the osteodentin matrix, thus limiting their capacity to contribute to reparative processes (Goldberg and Smith, 2004; Yin et al., 2021).
Similar to what happens in earlier stages of tooth development, pre-odontoblast differentiation requires signals from adjacent dental epithelium (Ruch et al., 1995; Thesleff and Hurmerinta, 1981). Thus, secretion of pre-dentin is essential for the complete differentiation of the inner enamel epithelium into ameloblasts. The epithelial-mesenchymal interactions that govern tooth development require the controlled secretion of signaling molecules, including BMPs, FGFs, Shh and Wnts (Jiménez-Rojo et al., 2012; Ruch et al., 1995), with transcription factors playing an essential role in the regulation of the paracrine mechanisms that underlie tooth formation.
Epiprofin/Sp6 is a zinc finger transcription factor that is expressed during embryonic development in the dental epithelium and in differentiating pre-odontoblasts, and in various tissues of ectodermal origin such as the matrix epithelium of the hair follicles, the distal epithelium of the urethra and the epithelium of the apical ectodermal ridge of the extremities (Nakamura et al., 2004). In line with this expression pattern, Epiprofin knockout (Epfn-/-) mice display alopecia, alterations in the external genitalia and limbs, develop supernumerary teeth, and present enamel hypoplasia (Nakamura et al., 2008). Mechanisms leading to defects in tooth number and enamel hypoplasia in Epfn-/- mice have been extensively analyzed during early developmental stages (Jiménez-rojo et al., 2010; Nakamura et al., 2008; Rhodes et al., 2021). Mice lacking Epfn develop a thinner dentin layer and it has been hypothesized that Epfn may play a role in odontoblast differentiation (Nakamura et al., 2008). However, the dentin phenotype in Epfn-/- mice has not yet been thoroughly studied. In this article, we contribute to the knowledge of the role of Epfn on dentinogenesis by describing the histological aspect of dentin and dentin-secreting odontoblasts in adult Epfn-/- mice and showing severe defects in early odontoblastic differentiation.
Results
Epiprofin deficiency disrupts the elongation of odontoblasts and results in abnormal dentin development
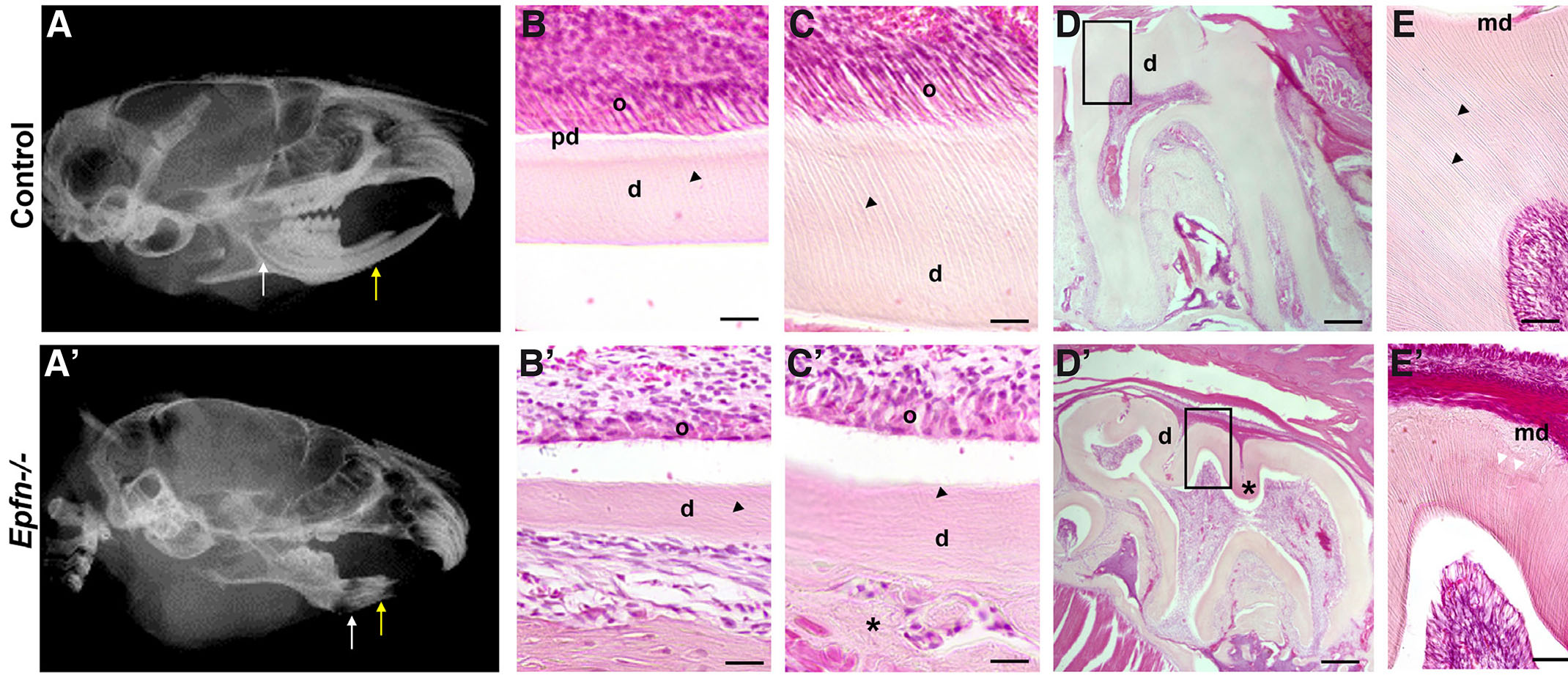
Radiographic images of Epfn-/- adult heads revealed dental radiolucency especially noticeable at the incisal region of incisors indicative of defects in tooth mineralization (Fig. 1 A-A’). Histological analysis showed that six-week-old, Epfn-/- mice display a thinner layer of dentin in incisors (Fig.1 B-B’, C-C’) and molars (Fig. 1 D-D’, E-E’). In control molars, coronal dentin comprised numerous parallel dentinal tubules that show ramifications at the mantle dentin area (Fig. 1E). In Epfn-/- molars, mantle dentin appears atubular and dentin tubules in circumpulpal dentin follow an irregular trajectory (Fig. 1E’). In addition, Epfn-/- dentin shows hypomineralized areas with interglobular dentin (Fig. 1E’).
Mouse incisors exhibit continuous growth and present all stages of odontoblast differentiation in a single dental piece, which makes them a very valuable model for the characterization of developmental disorders. The terminal differentiation of odontoblasts is characterized by the elongation and polarization of the cells. During this process, the nuclei are arranged opposite to the secretory pole. Odontoblasts then start synthesizing the components of pre-dentin. Thus, the first signs of odontoblast differentiation and pre-dentin/dentin deposition are observed in the apical area of incisors (Fig. 1B), whereas in the incisal area, close to the eruption site, odontoblasts appear as elongated cells in contact with mineralized dentin in which parallel dentin tubules are observed (Fig. 1C). In Epfn-/- incisors, odontoblasts are not correctly elongated and polarized, and dentin tubules are reduced in number and display irregular patterns (Fig. 1 B’, C’). In addition, in Epfn-/- mice, groups of round-shaped cells entrapped within an ectopic atubular matrix are observed at the external dentin surface in the incisal side of the incisors (Fig. 1C’).
Fig. 1. Dentin dysplasia in Epfn-/- mice.
Radiographic images of control (A) and Epfn-/- (A’) adult heads with white and yellow arrows indicating the apical and incisal regions of incisors, respectively. Detail of H&E-stained dentin at the apical region of control (B) vs Epfn-/- (B’) incisors showing defects in odontoblast polarization and dentin-matrix deposition in Epfn-/- mice. These defects persist in the incisal area of Epfn-/- incisors (C’) when compared to control incisors (C) in which odontoblasts appear polarized and dentin tubules are well-defined. Note defects in dentin tubules (black arrow tips) in Epfn-/- (B’, C’) vs control (B, C) and the presence of an ectopic matrix (asterisk) adjacent to the dentin layer at the incisal tip of Epfn-/- incisor (C'). Hematoxylin-eosin (H&E) staining of control (D, E) and Epfn-/- mouse molars (D’, E’). In control molars, dentin tubules (black arrow heads in D) appear parallel, whereas in Epfn-/- molars there are areas with disorganized tubules and hypomineralized areas (asterisk in D’) with interglobular dentin (white arrowheads in E’). Abbreviations: ab, alveolar bone; d, dentin; md, mantle dentin; o, odontoblasts; pd, pre-dentin. Scale bars: 100 µm in D, D’; 50 µm in E, E’; 25 µm in B, B’, C, C’.
Expression of Dsp/Dpp is absent in E19.5 Epfn-/- mouse molars
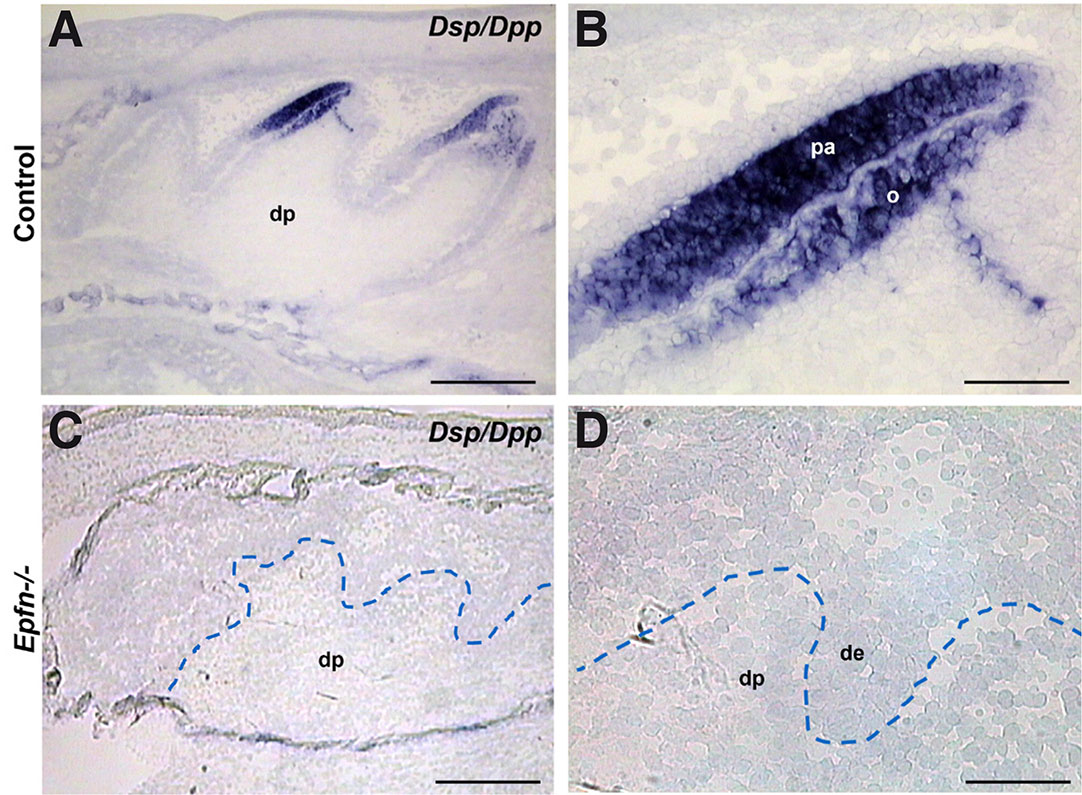
Epiprofin expression in dental mesenchyme starts at late bell stage in coincidence with the first signs of odontoblasts differentiation (E18.5-E19.5) (Nakamura et al., 2004). Based on that, we hypothesized that Epiprofin could be crucial for the initiation of pre-odontoblast differentiation. Consistently, we chose E19.5 with the aim of analyzing the effect of the loss of Epiprofin at early stages of odontoblastic differentiation. Thus, in E19.5 control molars (cell differentiation stage), differentiated odontoblasts are detected in the central region of the dental cusps. In this stage, the Dentin sialoprotein/Dentin phosphoprotein gene (Dsp/Dpp) is expressed both in early-differentiated odontoblasts and in pre-ameloblasts (Fig. 2 A,B). However, Epfn-/- E19.5 molars do not present dental cusps and expression of Dsp/Dpp is missing from both dental epithelium and mesenchyme (Fig. 2 C,D).
Fig. 2. Expression of the early odontoblast differentiation marker Dsp/Dpp is altered in dental differentiation process of odontoblasts is altered in Epfn-/- molars.
In situ hybridization for the Dsp/Dpp gene on cryostat sections of E19.5 control (A,B) and Epfn-/- (C,D) molars. Abbreviations: de, dental epithelium; dp, dental papilla; o, odontoblasts; pa, pre-ameloblasts. Scale bars: 250 µm in A,C; 50 µm in B,D.
Aberrant Type I Collagen (COL1) deposition in E19.5 Epfn-/- mouse molars
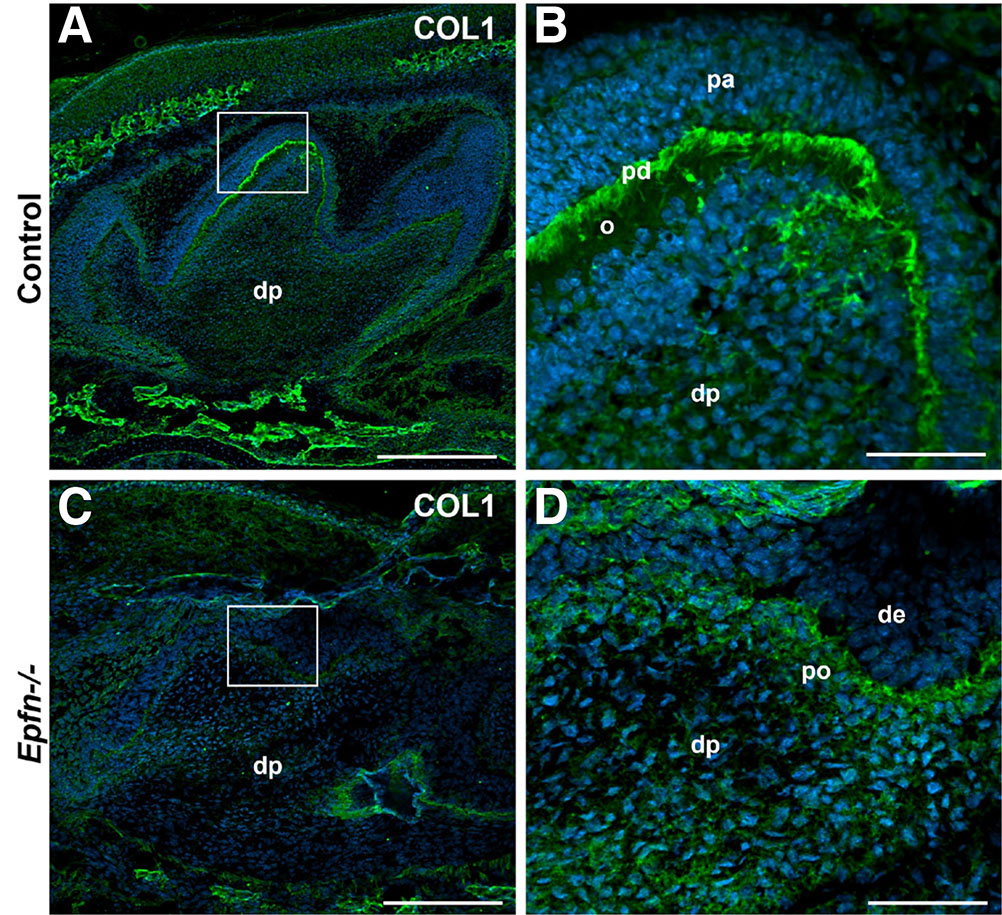
Type I Collagen (COL1) is the major organic component of mineralized dentin. Its synthesis increases considerably when odontoblasts become polarized and functional (Lesot, 1981). In E19.5 control molars, functional odontoblasts secrete matrix components such as COL1 mainly at the proximal region in contact with dental epithelium where pre-dentin is developing (Fig. 3 A,B). In turn, in Epfn-/- molars COL1 deposition is very scarce (Fig. 3 C,D) and there is no clear pre-dentin layer, as the mesenchymal cells in contact with dental epithelium do not show signs of polarization.
Fig. 3. Fig. 3. Defective Type I Collagen expression in Epfn-/- molars.
Immunofluorescence against Type I Collagen (COL1, in green) on cryostat sections of E19.5 control (A,B) and Epfn-/- (C,D) molars. Nuclei (DAPI) are stained in blue. Abbreviations: de, dental epithelium; dp, dental papilla; o, odontoblasts; pa, pre-ameloblasts; pd, pre-dentin; po, pre-odontoblasts. Scale bars: 250 µm in A,C; 50 µm in B,D.
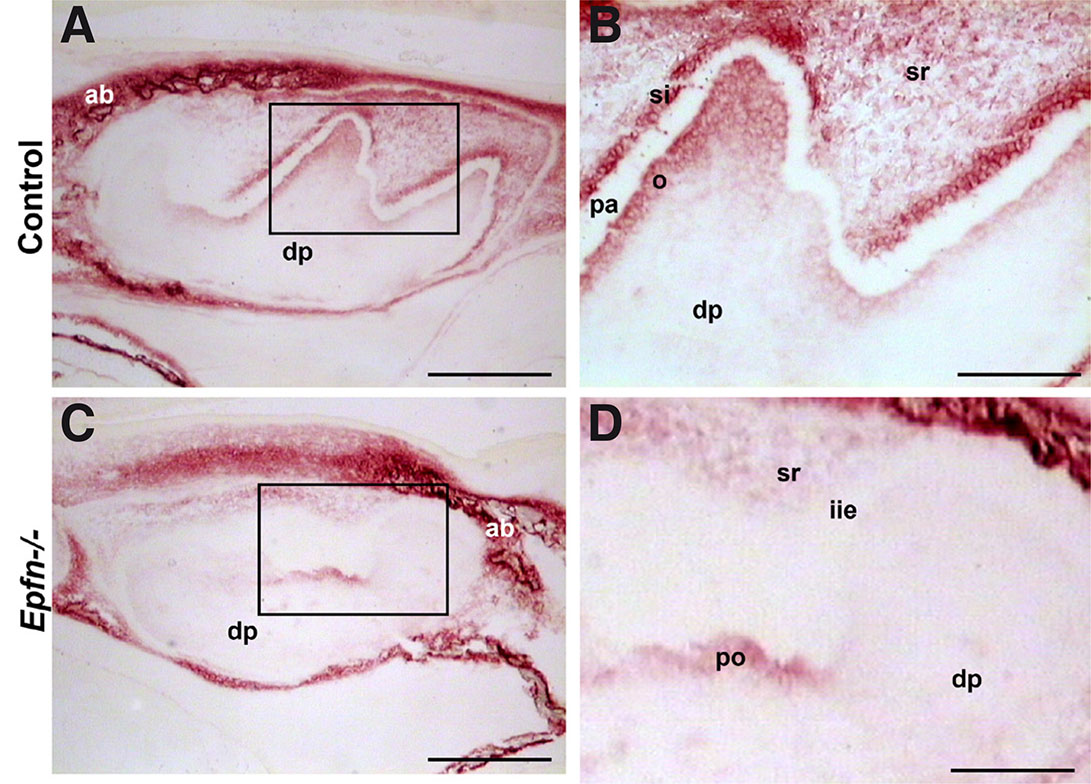
Alkaline phosphatase activity decreases in Epfn-/- molars
The presence of interglobular dentin suggested defects in dentin mineralization in Epfn-/- molars. Since alkaline phosphatase is related to the onset of mineralization (Väkevä et al., 1990), we analyzed the activity of this enzyme in molars at late bell stage. In E19.5 wild-type molars, enzyme activity was detected in alveolar bone, stellate reticulum, stellate reticulum, odontoblasts, and dental mesenchyme cells underlying odontoblasts, whereas pre-ameloblasts were negative (Fig. 4 A,B). In the case of Epfn-/- molars, alkaline phosphatase activity appeared normal in the alveolar bone, but was decreased in the enamel organ, pre-odontoblasts and in the subjacent subodontoblastic layer (Fig. 4 C,D).
Fig. 4. Alkaline phosphatase activity decreases in E19.5 Epfn-/- molars.
Detection of alkaline phosphatase activity in cryostat sections of E19.5 control (A,B) and Epfn-/- (C,D) molars. Alkaline phosphatase activity appears reduced in the pre-odontoblastic region in Epfn-/- molars. Note the absence of stratum intermedium in the Epfn-/- enamel organ. Abbreviations: ab, alveolar bone; dp: dental papillae; iee: inner enamel epithelium; o: odontoblasts; pa: pre-ameloblasts; po, pre-odontoblasts; si, stratum intermedium; sr, stellate reticulum. Scale bars: 250 µm in A,C; 100 µm in B,D.
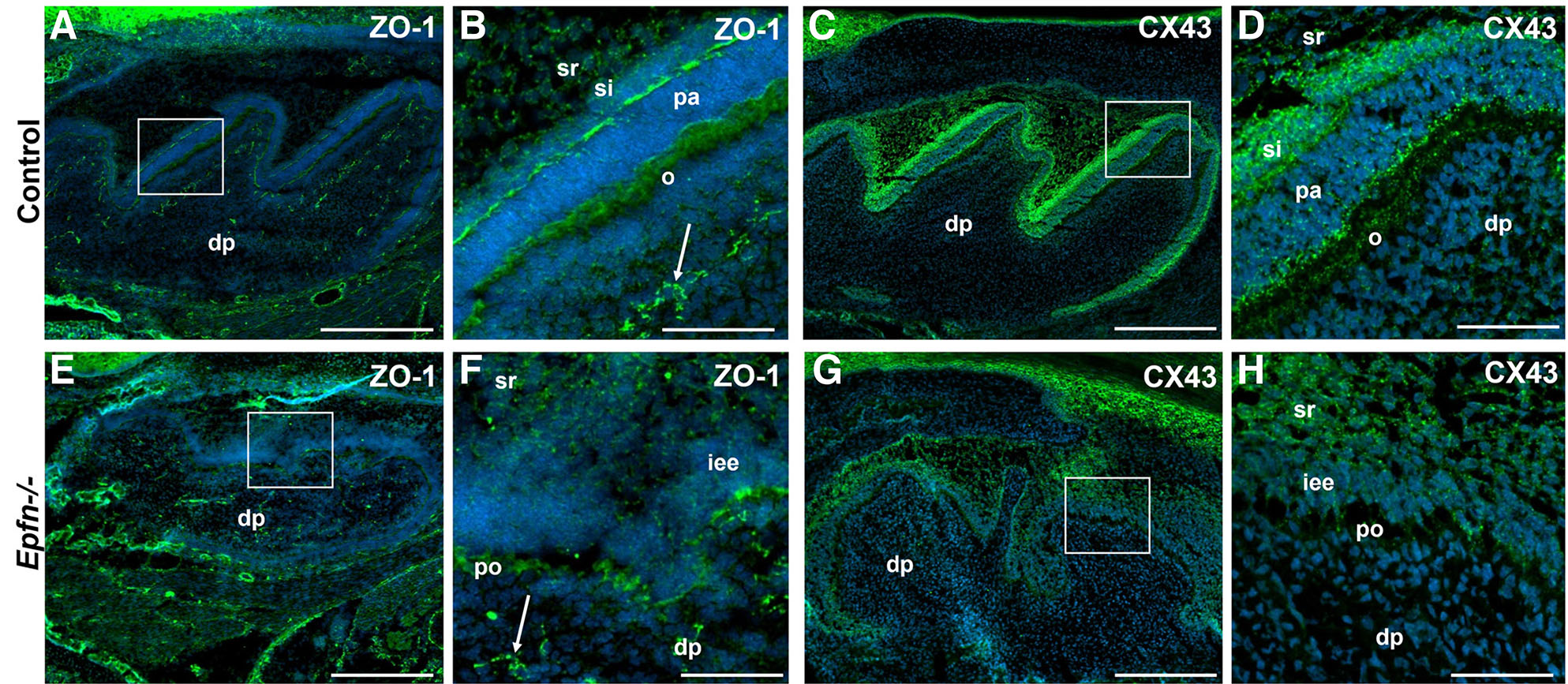
Defective tight and gap junctions in E19.5 Epfn-/- molars
As differentiating odontoblasts secrete the first layers of mantle dentin, they become more closely packed and develop numerous gap and adherents junctions (Arana-Chavez and Massa, 2004). Tight junctions establish an intercellular barrier and participate in the generation and maintenance of cell polarization (Anderson, 2001; Arana-Chavez and Massa, 2004). In order to study whether the incorrect polarization of odontoblasts in Epfn-/- mice is related to an alteration of tight junctions, we studied the expression of ZO1 (Zonula Occludens 1). In E19.5 control molars, ZO1 protein is expressed in both the distal and proximal poles of pre-ameloblasts, in the apical pole and lateral surfaces of differentiated odontoblasts and in the endothelial cells of the blood capillaries of the dental papilla (Fig. 5 A,B). In Epfn-/- molars, ZO1 is detected in endothelial cells and pre-odontoblasts, although expression is markedly lower when compared to odontoblasts in control molars (Fig. 5 E,F).
Fig. 5. ZO1 and CX43 expression is decreased in E19.5 Epfn-/- molars.
Immunofluorescence against ZO1 antigen (A,B) in green and CX43 (C,D) in green in E19.5 control molars. Expression of ZO1 (E,F) and CX43 (G,H) in Epfn-/- molars. Nuclei (DAPI) are stained in blue. ZO1 expression in Epfn-/- molars is detected in endothelial cells (white arrow in F) and pre-odontoblasts, although in the latter with less intensity and in a less ordered manner than in control molars. In the case of CX43, a decrease in the expression of the protein is again observed in the pre-odontoblasts of the Epfn-/- molar compared to that observed in odontoblasts of the control molar. Abbreviations: dp, dental papilla; iee, inner enamel epithelium; o, odontoblasts; pa, pre-ameloblasts; po, pre-odontoblasts; si, stratum intermedium; sr, stellate reticulum. Scale bars: 250 µm in A,C; 50 µm in B,D.
Connexin43 (CX43) is strongly expressed in the enamel organ of wild type mice, including all four epithelial compartments (pre-ameloblasts, stratum intermedium, stellate reticulum and outer enamel epithelium) (Fig. 5 E,G). Regarding dental mesenchymal component, CX43 is limited to discrete spots at the distal region of odontoblasts (Fig. 5 E,G). In Epfn-/- molars, the inner enamel epithelium does not show signs of differentiation and stratum intermedium is absent. Thus, CX43 expression is dramatically reduced in both epithelium and pre-odontoblasts from Epfn-/- molars (Fig. 5 F,H). These results confirm that in the mutant mouse molars, the process of terminal differentiation of odontoblasts is altered, which is reflected in an inverted polarization in some of the adult odontoblasts and an incomplete secretion of dentin matrix components.
Discussion
Dentin is a mineralized hard tissue synthetized by odontoblasts, which arise from dental mesenchymal cells at the bell stage of tooth development. As they differentiate, odontoblasts become polarized and develop long apical processes that get trapped inside narrow channels known as dentin tubules (Goldberg, 2011). Due to the presence of odontoblastic cell processes inside the mineralized matrix, dentin maintains the ability to respond to certain stimuli and activate dentin-matrix secretion if required. Epfn-/- adult mice present dysplastic dentin in molars and incisors with dentin tubules following an irregular architecture. Defects in odontoblasts differentiation and their organization have been associated with the formation of a defective dentin tubular system in transgenic mice with dysplastic and hypomineralized dentin (Lézot et al., 2002; Lu et al., 2007). Defective dentin tubules may result in a failure to deliver calcium or phosphorus to sites of mineralization, contributing to the development of a hypomineralized dentin (Lu et al., 2007).
Although Epfn is expressed in dental epithelium from early stages of tooth development, its expression in dental mesenchyme begins at E18.5 in coincidence with the first signs of pre-odontoblasts differentiation (Nakamura et al., 2004). This suggests that mesenchymal Epfn plays a role in the regulation of early odontoblastic differentiation. In fact, we have observed a severe downregulation in markers for early odontoblast differentiation, such as alkaline phosphatase activity, Dsp/Dpp expression and Type I Collagen, in Epfn-/- mice. Altogether, our data support an incomplete differentiation of odontoblasts in the absence of Epfn. Alteration in the expression of molecules such as Nestin or BMP4 (Bone Morphogenetic Protein 4) in dental papilla from Epfn-/- mouse molars at cap stage and early bell stage have been previously reported (Ibarretxe et al., 2012; Nakamura et al., 2008). However, as previously mentioned, Epfn is not expressed in dental papilla at early stages, suggesting that the dysregulation caused by Epfn absence is a consequence of an abnormal epithelial-mesenchymal crosstalk.
During cell polarization, intercellular junctions are established in a defined pattern (Arana-Chavez and Massa, 2004). At early differentiation stages, the number and size of gap-type junctions between odontoblasts increases at locations where the distal junctional complex is actively developing (Goldberg and Smith, 2004). Epfn-/- molars show alterations in the expression of elements of these junctions, such as ZO1 and CX43, which may account for the defects in odontoblast differentiation. In line with our results, defects in the expression of ZO1 in odontoblasts have been recently found in other transgenic mouse models in which dentin deposition and odontoblasts are severely affected (Liang et al., 2023). It has been also described that CX43-containing gap junctions facilitate Ca2+-induced odontoblastic differentiation of human dental pulp stem cells (DPSCs) (Li et al., 2015).
Interestingly, in Epfn-/- mice an ectopic matrix at the tip of the incisors is deposited. Its amorphous structure and the presence of round-shaped cells trapped within it are indicative of an osteodentin-like matrix. A similar phenotype has been previously observed in knockout mice with dentin defects in which the expression of odontoblasts specific markers such as Dsp/Dpp are altered and odontoblasts are redirected towards an osteoblastic fate (Zhang et al., 2019).
Taken together, our results point to Epfn as a master regulator of cell fate switch of oral mesenchymal stem cells towards dentin-secreting odontoblasts. Thus, when Epfn is absent, mesenchymal stem cells from mouse incisors may switch towards an osteoblastic differentiation, leading to the secretion of an osteoid-like matrix. Another possible explanation for the deposition of the atubular ectopic matrix at the tip of Epfn-/- incisors could be the formation of an amorphous tertiary dentin with osteodentin morphology as a response to external insults. Since the Epfn-/- mice completely lack enamel, dentin is fully exposed and is more susceptible to abrasion, which might underlie the formation of reparative dentin matrix (Nakamura et al., 2008).
In humans, dental defects arise as isolated or syndromic congenital anomalies (Klein et al., 2013). In the case of dentin, congenital anomalies in form of mutations in genes encoding for dentin-matrix proteins such as DSPP, COL1 and MMP20 have been described (Barron et al., 2008; de La Dure-Molla et al., 2015; Hart and Hart, 2007; Kovacs et al., 2021). However, there is still a large number of cases in which the genetic cause remains unknown.
Recently, a de novo mutation in human EPFN/SP6 has been identified in a family with hereditary enamel defects (hypoplastic Amelogenesis Imperfecta) (Kim et al., 2021). Hereby, we show that early differentiation of odontoblasts and dentin formation is affected in Epfn-/- mice. Thus, our data suggest that possible mutations in human EPFN/SP6 may also account for genetic dentin disorders in which the genetic cause has not been identified yet.
Materials and Methods
Mice
Control Epfn+/+ and Epfn-/- deficient mice were generated as previously described (Nakamura et al., 2008). All mice were maintained in compliance with the National Institutes of Health (NIH, Bethesda, USA) guidelines on the Use of Laboratory and Experimental Animals. The animal experimentation protocol was approved by the National Institute of Dental and Craniofacial Research (NIDCR) Animal Care and Use Committee (License number ASP16-796).
Tissue preparation and X-ray analysis
Embryonic day 19.5 (E19.5) mice were sacrificed by decapitation and the heads were dissected out and fixed in 4% formaldehyde (FA) overnight at 4°C. Then they were cryoprotected with 30% sucrose in PBS overnight and embedded in Tissue-Tek OCT compound (127217, Sakura Finetek Europe) for cryostat (8 µm) sectioning.
Six-week-old mice were anaesthetized with isoflurane were perfused transcardially with PBS. Perfusion fixation was performed with 4% FA in 0.1 M phosphate buffer. Heads were further fixed 1 hour at room temperature in 4% FA and then overnight at 4°C. Heads were placed in 70% ethanol before X-ray analysis (Faxitron X-Ray System) and then decalcified in 10% EDTA (in PBS) for 4-6 weeks and processed for paraffin embedding, microtome sectioning (4 µm) and histological analysis.
Detection of endogenous alkaline phosphatase activity
Cryostat sections were allowed to dry at room temperature for about 20 min and then fixed in cold methanol and a cold methanol:acetone mixture (1:1) for 10 min in both cases. After washing in PBS the samples were incubated for 20 min with a mixture composed of 0.6% MgCl2, 5% veronal (32002, Sigma), α-naphthylphostat (N5602, Sigma) at 1mg/ml and Fast-Red (368881, Sigma) at 5 mg/ml. Cells showing alkaline phosphatase activity will appear reddish in color (Dinkelaker and Marschner, 1992).
In situ hybridization on tissue sections
Digoxigenin-11-UTP-labeled single-stranded RNA probe for Dsp/Dpp was prepared using a DIG RNA labeling kit (11175025910, Roche) according to the manufacturer's instructions. RNA in situ hybridization was performed in cryostat sections from E19.5 heads as previously described (Nakamura et al., 2004).
Immunofluorescence
Cryostat sections from E19.5 mouse heads were stained with antibodies against type I Collagen (COL1), Connexin43 (CX43) and Zonula Ocludens-1 (ZO1). Briefly, sections were allowed to dry for about 30 min and then fixed in cold (-20°C) methanol for 10 min and with methanol:acetone (1:1) for 10 min. Sections were washed three times in PBS and then blocked with 10% fetal bovine serum in PBS for 1 hour. Then, tissues were incubated overnight with rabbit polyclonal anti-Collagen I (1:160; ab34710, Abcam), anti-Connexin43 (1:200; ab11370, Abcam) or anti-ZO1 (1:200; ab59720, Abcam) antibodies at 4ºC. Next day, samples were washed with PBS, and subsequently incubated with a Cy2 Goat Anti-Rabbit IgG secondary antibody (1:200, ab6940, Abcam). Finally, sections were incubated with DAPI (4′,6-Diamidino-2-phenylindole) 1 μg/ml (D9542, Sigma) and mounted with Fluoromount G (00-4958-02, Invitrogen).
Acknowledgements
An appreciation to Dr. Yoshihiko Yamada (Laboratory of Cell and Developmental Biology, National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, Maryland, USA) who sadly passed away in December 2019 and extensively contributed to the identification of Epfn/Sp6 gene as well as the generation of Epfn-related mouse models. We thank Dr. Taduru Sreenath (NIDCR, Bethesda) for the Dsp/Dpp probe.
This work has been supported by the Intramural Research Program of NIDCR, (NIH, USA) and by institutional funds from the University of the Basque Country (UPV/EHU; GIU20/050) and the Basque Government (GV/EJ; IT1751-22).
Abbreviations
COL1, Type I Collagen ; CX43, Connexin43 ; d, dentin ; dp, dental papilla ; DSP/DPP, Dentin sialoprotein/Dentin phosphoprotein ; E19.5, Embryonic Day 19.5 ; Epfn, Epiprofin ; iie, inner enamel epithelium ; o, odontoblasts ; pa, pre-ameloblasts ; pd, pre-dentin ; po, pre-odontoblasts ; si, stratum intermedium ; sr, stellate reticulum ; ZO1, Zonula Occludens 1 ;References
Anderson J. M. (2001). Molecular Structure of Tight Junctions and Their Role in Epithelial Transport. Physiology 16: 126-130.
Arana-Chavez V. E., Massa L. F. (2004). Odontoblasts: the cells forming and maintaining dentine. The International Journal of Biochemistry & Cell Biology 36: 1367-1373.
Barron M. J., McDonnell S. T., MacKie I., Dixon M. J. (2008). Hereditary dentine disorders: dentinogenesis imperfecta and dentine dysplasia. Orphanet Journal of Rare Diseases 3: 31.
de La Dure-Molla M., Philippe Fournier B., Berdal A. (2015). Isolated dentinogenesis imperfecta and dentin dysplasia: revision of the classification. European Journal of Human Genetics 23: 445-451.
Dinkelaker B., Marschner H. (1992). In vivo demonstration of acid phosphatase activity in the rhizosphere of soil-grown plants. Plant and Soil 144: 199-205.
Goldberg M. (2011). Dentin structure composition and mineralization. Frontiers in Bioscience E3: 711-735.
Goldberg M., Smith A. J. (2004). C ells and E xtracellular M atrices of D entin and P ulp: A B iological B asis for R epair and T issue E ngineering . Critical Reviews in Oral Biology & Medicine 15: 13-27.
Hart P. S., Hart T. C. (2007). Disorders of Human Dentin. Cells Tissues Organs 186: 70-77.
Ibarretxe G., Aurrekoetxea M., Crende O., Badiola I., Jiménez-Rojo L., Nakamura T., Yamada Y., Unda F. (2012). Epiprofin/Sp6 regulates Wnt-BMP signaling and the establishment of cellular junctions during the bell stage of tooth development. Cell and Tissue Research 350: 95-107.
Jiménez-Rojo L., Granchi Z., Graf D., Mitsiadis T. A. (2012). Stem Cell Fate Determination during Development and Regeneration of Ectodermal Organs. Frontiers in Physiology 3: 107.
Jiménez-Rojo L., Ibarretxe G., Aurrekoetxea M., de Vega S., Nakamura T., Yamada Y., Unda F., (2010). Epiprofin/Sp6: a new player in the regulation of tooth development. Histology and histopathology 25: 1621-1630.
Kim Y. J., Lee Y., Zhang H., Song J.S., Hu J. C.C., Simmer J. P., Kim J.W. (2021). A Novel De Novo SP6 Mutation Causes Severe Hypoplastic Amelogenesis Imperfecta. Genes 12: 346.
Klein O. D., Oberoi S., Huysseune A., Hovorakova M., Peterka M., Peterkova R. (2013). Developmental disorders of the dentition: An update. American Journal of Medical Genetics Part C: Seminars in Medical Genetics 163: 318-332.
Kovacs C. S., Chaussain C., Osdoby P., Brandi M. L., Clarke B., Thakker R. V. (2021). The role of biomineralization in disorders of skeletal development and tooth formation. Nature Reviews Endocrinology 17: 336-349.
Lézot F., Descroix V., Mesbah M., Hotton D., Blin C., Papagerakis P., Mauro N., Kato S., MacDougall M., Sharpe P., Berdal A. (2002). Cross-Talk Between Msx/Dlx Homeobox Genes and Vitamin D During Tooth Mineralization. Connective Tissue Research 43: 509-514.
Li S., He H., Zhang G., Wang F., Zhang P., Tan Y. (2015). Connexin43-containing gap junctions potentiate extracellular Ca2+-induced odontoblastic differentiation of human dental pulp stem cells via Erk1/2. Experimental Cell Research 338: 1-9.
Liang T., Smith C. E., Hu Y., Zhang H., Zhang C., Xu Q., Lu Y., Qi L., Hu J. C.C., Simmer J. P. (2023). Dentin defects caused by a Dspp−1 frameshift mutation are associated with the activation of autophagy. Scientific Reports 13: 6393.
Lu Y., Ye L., Yu S., Zhang S., Xie Y., McKee M. D., Li Y. C., Kong J., Eick J. D., Dallas S. L., Feng J. Q. (2007). Rescue of odontogenesis in Dmp1-deficient mice by targeted re-expression of DMP1 reveals roles for DMP1 in early odontogenesis and dentin apposition in vivo. Developmental Biology 303: 191-201.
Nakamura T., de Vega S., Fukumoto S., Jiménez L., Unda F., Yamada Y. (2008). Transcription Factor Epiprofin Is Essential for Tooth Morphogenesis by Regulating Epithelial Cell Fate and Tooth Number. Journal of Biological Chemistry 283: 4825-4833.
Nakamura T., Unda F., de-Vega S., Vilaxa A., Fukumoto S., Yamada K. M., Yamada Y. (2004). The Krüppel-like Factor Epiprofin Is Expressed by Epithelium of Developing Teeth, Hair Follicles, and Limb Buds and Promotes Cell Proliferation. Journal of Biological Chemistry 279: 626-634.
Rhodes C. S., Yoshitomi Y., Burbelo P. D., Freese N. H., Nakamura T., Chiba Y., Yamada Y. (2021). Sp6/Epiprofin is a master regulator in the developing tooth. Biochemical and Biophysical Research Communications 581: 89-95.
Ruch J. V., Lesot H., Bègue-Kirn C., (1995). Odontoblast differentiation. The International journal of developmental biology 39: 51-68.
Smith A. J., Cassidy N., Perry H., Bègue-Kirn C., Ruch J. V., Lesot H., (1995). Reactionary dentinogenesis. The International journal of developmental biology 39: 273-280.
Thesleff I., Hurmerinta K. (1981). Tissue Interactions in Tooth Development. Differentiation 18: 75-88.
Väkevä L., Mackie E., Kantomaa T., Thesleff I. (1990). Comparison of the distribution patterns of tenascin and alkaline phosphatase in developing teeth, cartilage, and bone of rats and mice. The Anatomical Record 228: 69-76.
Yin J., Xu J., Cheng R., Shao M., Qin Y., Yang H., Hu T. (2021). Role of connexin 43 in odontoblastic differentiation and structural maintenance in pulp damage repair. International Journal of Oral Science 13: 1.
Zhang X., Shi C., Zhao H., Zhou Y., Hu Y., Yan G., Liu C., Li D., Hao X., Mishina Y., Liu Q., Sun H. (2019). Distinctive role of ACVR1 in dentin formation: requirement for dentin thickness in molars and prevention of osteodentin formation in incisors of mice. Journal of Molecular Histology 50: 43-61.